DF is not a well-defined chemical entity, but a term that in both human and animal nutrition literature has been defined by the methods applied for its analysis. DF is the sum of non-starch polysaccharides (NSP) and lignin. The main polysaccharides of NSP are cellulose, and a wide variety of non-cellulosic polysaccharides - β-glucan, arabinoxylans, xylans, pectins, to mention the major ones. Other carbohydrates that have digestibility properties similar to NSP are non-digestible oligosaccharides and resistant starch. Some non-digestible carbohydrates may be prebiotic, i.e. carbohydrates that: “beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of health-promoting bacteria in the intestinal tract thus improving the host’s intestinal physiology”.
A common feature of all DF sources is the ability to swell, hold water in the cell wall matrix, and to increase viscosity when exposed water (Figure 1). However, while all DF sources swell and hold water the viscous properties depend on the type and chemical nature of the polysaccharides making up the DF fraction. For instance, sugar beet pulp will primarily increase the water binding capacity of digesta whereas the viscosity elevating properties of sugar beet pulp is relatively low. Contrary to that, β-glucan will to a larger extent solubilise from the cell wall matrix and raise luminal viscosity. However, far the largest effects on luminal viscosity are obtained by isolated DF sources such as β-glucan, pectin’s, guar gums etc.
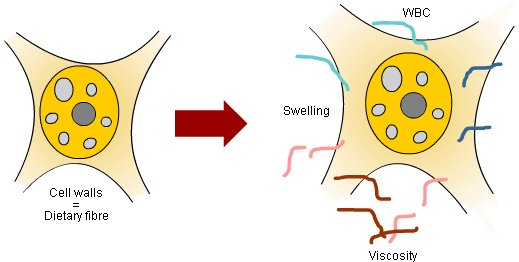
Figure 1. The hydration properties are characterised by the swelling capacity, solubility, and water binding capacity (WBC). The first part of the solubilisation process of polymers is swelling in which incoming water spreads the macromolecules until they are fully extended and dispersed. The majority of polysaccharides give viscous solutions if dissolved in water. The viscosity is dependent on the chemical structure, the molecular weight of the polymer, and concentration.
DF will influence the digestion and absorption processes along the gastrointestinal tract (Figure 2). Soluble DF will raise luminal viscosity thereby prolonging gastric emptying and interfering with the digestion processes in the small intestine by hindering the contact between the substrate and digestive enzymes and by slowing down the movements of hydrolytic products of the digestion processes.
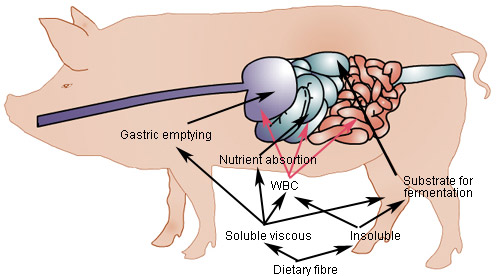
Soluble as well as insoluble DF will provide substrate for fermentation by the microflora in the large intestine. Soluble DF is readily fermentable; the major part is broken down in caecum and proximal colon while insoluble DF is be degraded at more distal locations. While DF in general increase the flow of carbohydrates to the large intestine and thereby stimulate the entire microbial population there are only few non-digestible carbohydrates that have the ability specifically to stimulate beneficial microbial groups. Fructose containing oligo- and polysaccharides, however, has this ability as they stimulate lactic acid bacteria (Lactobacillus spp. together with Bifidobacterium spp.). It is a generally held belief that stimulation of lactic acid bacteria is beneficial as attachment of these harmless bacteria to the mucosa may protect the animals from gut infection. Increased microbial fermentation will also stimulate the formation of short-chain fatty acids in the large intestine, which will reduce luminal pH. Fermentable but particularly resistant DF will increase the bulk in the large intestine and in faeces thereby reducing the total tract digestibility.
There is at present conflicting evidence as to whether DF exerts beneficial or detrimental influence of gut health. For example, an almost DF free rice based diet supplemented with animal protein has been shown to reduce the susceptibility to enteric disorders including post weaning enteric disorder, and swine dysentery. The same was the case with DF from the outer hull of barley, rich in insoluble DF, whereas feeding barley meal and guar gum, high in soluble DF, have been associated with increased susceptibility to post weaning enteric disorders, swine dysentery or porcine intestinal spirochaetosis. However, recently it has been shown that pigs fed a diet based on dried chicory roots and sweet lupins were completely protected against the infection with Brachyspira hyodysenteria after experimental challenge. Detailed microbial characterisation revealed that pigs fed the dried chicory roots and sweet lupins diet had a higher proportion of Bifidobacterium thermoacidophilum and Megasphaera elsdenii, which were thought to inhibited B. hyodysenteriae from being established.



