Autogenous vaccines have been given various names (autologous vaccine, herd or emergency vaccine, custom-made vaccine, tailor-made vaccines, etc.) and definitions. However, since their use and production in veterinary medicine are regulated, we must consider the legal definition:"inactivated immunological veterinary medicinal products which are manufactured from pathogens and antigens obtained from an animal or animals in an epidemiological unit and used for the treatment of that animal or those animals in the same epidemiological unit or for the treatment of an animal or animals in a unit having a confirmed epidemiological link" (REGULATION (EU) 2019/6 of 11 December 2018 on veterinary medicinal products which came into effect in January 2022).
Thus, autogenous vaccines are a type of vaccine that is:

- Intended for a farm, or group of epidemiologically related farms
- Inactivated
- Preventive as vaccines
The incorporation of farm-specific pathogenic strains, whether they are bacteria or viruses, makes them highly specific and versatile. It is possible to include several different pathogens or different antigenic variants of the same pathogen in the same autogenous vaccine, something that is usually done in multifactorial processes.
They are not commercial products. They are custom-made vaccines produced only on request and under veterinary prescription for a wide variety of animal species and pathogens and in varying quantities. This allows their composition to be updated to include new strains that appear on the farm.
As inactivated vaccines they are generally formulated with adjuvants, necessary to boost the immune response, and at least n mammals and birds they are almost always applied parenterally. Likewise, inactivation makes them very safe since they can never reproduce the disease they are intended to control.
When are autogenous vaccines indicated?
Again, we will have to abide by the legal aspects beyond the technical indications, but in general, we can say that they fill the gaps left by commercial vaccines and can be used in the following cases:
- When commercial vaccines are not available for that animal species and that indication. A clear example would be autogenous vaccines against Staphylococcus hyicus, Brachyspira hyodysenteriae, or Metamycoplasma hyosynoviae.
- When the licensed vaccines do not include the antigenic variants present in our farm and there is no cross-protection between them. The variants can be determined by the serotype, toxins, or virulence factors they carry. This would be the case for Streptococcus suis, Actinobacillus pleuropneumoniae, or Glaesserella parasuis among others.
- If a suitable commercial vaccine is available, an autogenous vaccine could only be used if the former do not provide the expected protection and the lack of efficacy has been demonstrated and reported.
Autogenous vaccine production process
The complete protocol (Table 1) involves a series of steps in which several actors are involved:
- The veterinarian. They are responsible for detecting the clinical process on the farm, collecting the samples, prescribing the autogenous vaccine, and supervising or executing its administration.
- The diagnostic laboratory. It will carry out the analysis and isolation of the pathogenic strains.
- The autogenous vaccine manufacturer laboratory. It can be the same laboratory as the diagnostic lab or a different one. It must be authorized and will be in charge of designing the composition of the autogenous vaccine (in coordination with the veterinarian and according to the diagnostic results) and of manufacturing it.
Table 1. Autogenous vaccine.production process
| Actor | Step | Description | |
|---|---|---|---|
|
|
Veterinarian |
Presence of sick animals with evident clinical signs |
The veterinarian makes a clinical diagnosis |
|
|
Veterinarian | Sample collection | Samples sent to the laboratory |
|
|
Diagnostic laboratory | Laboratory diagnostics | Differential diagnostic, isolation of strains and strain characterization |
|
|
Autogenous vaccine manufacturing laboratory | Autogenous vaccine design | Define which strains, serotypes, etc. have to be included. |
|
 |
Veterinarian | Order | Always under veterinary prescription |
|
|
Autogenous vaccine manufacturing laboratory | Manufacture | Following standardized production protocols |
|
|
Autogenous vaccine manufacturing laboratory | Quality control | Composition, inactivation, and sterility of the final injectable product |
|
|
Veterinarian | Shipment and administration | Sent to owner or veterinarian, administered according to established protocol. |
Key points for a high probability of success with autogenous vaccines
1. Diagnostic focus
It will be different depending on the pathological process but it starts by providing instructions for a correct on-farm sampling. The laboratory should recommend the type and quantity of samples to be collected and the type of animals to be sampled. It is important to analyze more than one batch of animals or batches from different production stages. A complete differential laboratory diagnosis should always be performed to identify all those agents that could cause the clinical process observed, taking into account that many pathologies are multifactorial, and underlying processes may be prsent. For autogenous vaccines it is necessary to identify antigenic variants whenever possible and to isolate in culture all identified strains and characterize them adequately. The strains will be preserved so that batches of autovaccine can be produced as required.
2. Manufacturing process
It will depend on the know-how of each laboratory, but in any case it must be specifically adapted to a large number of different bacterial or viral isolates, serotypes, toxins, or their combinations. There are a series of steps, each of them with determining variables for the quality and efficacy of the prepared batch. It will take between 4 and 6 weeks.
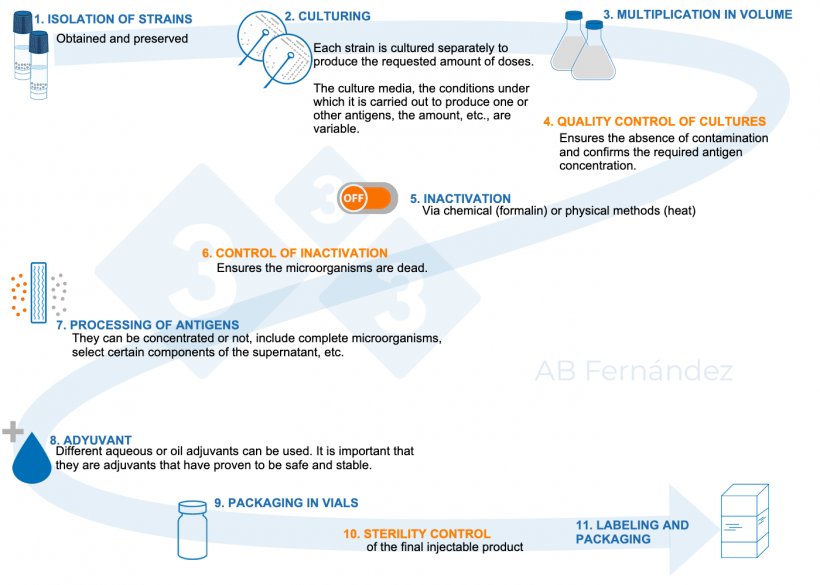
Figure 1. Schematic diagram of the manufacture of an autogenous vaccine.
3. Administration protocol
Like any other vaccine, autogenous vaccines should be integrated into the farm's sanitary control program, which should be properly and continuously implemented following the laboratory and veterinarian's indications, in order to see results in the medium and long term.
In conclusion, autogenous vaccines can be a very useful tool, and sometimes an essential one, within the strategies of health control and welfare management. As long as they are prescribed after a correct and complete diagnosis and with an adequate administration on the farm, they are highly effective in the prevention of many infectious processes, as well as cost-effective.