Consider the big picture
E. coli is a ubiquitous and complex bacterium found on all pig farms. The term colibacillosis refers broadly to any disease or condition caused by E. coli. Managing colibacillosis in pigs requires an understanding of the biology of the many types of E. coli and also the conditions under which they are capable of causing disease. We can gain some broader insights on control methods if we briefly back away from E. coli specifically and consider the big picture of infectious diseases

How to succeed as a pathogen
A helpful outline of steps that a pathogen must follow in order to cause disease appeared in an introduction to a book on bacterial pathogenicity (Gyle CL, Prescott JF, 2010). These steps provide a framework for thinking about diagnostic and prevention strategies.
The steps necessary for succeeding as a pathogen are:
- Enter and/or attach to body
- Evade host defenses
- Multiply
- Damage the host
- Transmit to the next body
The good news is that interrupting one or more of the steps can be the key to an effective control strategy. For colibacillosis, the first step, entry and attachment, is driven by well-defined receptors for fimbrial adhesins that appear and disappear in pigs at different ages (or not at all). The second and third steps, evading host defenses and multiplying, can be influenced by something as common and simple as hypothermia in newborn pigs, since colostrum intake and absorption as well as flushing of pathogenic bacteria are reduced with cold stress (Blecha F, Kelley KW, 1981). The fourth step, damaging the host, is driven by toxins that may or may not be produced by specific strains of E. coli, depending on the presence or absence of virulence genes. Evaluating the steps against the available control methods can provide focus and understanding as to what control measure are intended to achieve relative to the tactics used by the pathogen.
Five production input model of disease causation
Another way of looking at this same concept is illustrated in figure 1 using a Fishbone (or cause and effect) Diagram developed by Dr. Kent Schwartz from Iowa State University. This line of thinking pulls in five interconnected production input ‘causes’ of neonatal enteric colibacillosis in pigs. This is helpful because it draws the focus beyond just the disease-causing agent to include a broader set of risk factors contributing to the disease.
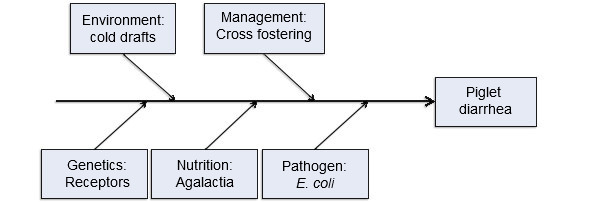
Figure 1. Five production input model of disease causation. Figure courtesy of Dr. Kent Scwartz, Iowa State University
Simple math
I propose a simple equation as another way to approach infectious disease generally and colibacillosis specifically:
Infectious Disease = (susceptibility)(dose)(virulence)

This equation allows us to focus on what we are trying to accomplish with our control strategy. If we start at the end of the equation, virulence, we realize this variable is, in fact, highly variable (Blecha F, Kelley KW, 1986). Figure 2 illustrates the spectrum of virulence genes identified over the past three years among porcine E. coli isolates at the Minnesota Veterinary Diagnostic Laboratory. (A word of caution: These data are from clinical enteric disease case submissions and as such are, at best, passive surveillance that may not represent true field prevalence.) With respect to control strategies, we really have no control over this variable in the field, but it is important information because we need to know what we are trying to control.
E. coli virulence factor prevalence from porcine cases
at the Minnesota Veterinary Diagnostic Laboratory, July 2012-June 2015
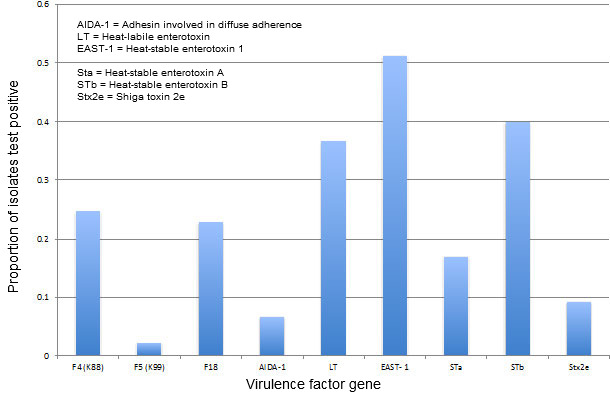
Figure 2. E. coli virulence factor prevalence. Data courtesy of Mary Thurn, University of Minnesota
The second variable, dose, is one we have more control over. However, it has proven to be very challenging to execute effective cleaning and disinfecting regimens to eliminate F18 E. coli in particular from facilities. That’s not to say we shouldn’t try, but we need to pick our battles carefully and focus our energies where we have the best chance to win.
The first variable, susceptibility, is an important area of opportunity for E. coli control. Receptors in the pig gut for F18 E. coli don’t completely develop until around 3 weeks-of-age, so young pigs are not susceptible to infection (Coddens A et al., 2007). Nor are they susceptible to immunization against F18 E. coli, so the challenge is to vaccinate pigs late enough for immunity to develop but early enough to get ahead of field exposure. Later weaning can be a management tool to help with this paradox (McLamb BL et al., 2013). Additionally, the receptor for the F18 fimbria is absent from or has been bred out of some lines of pigs, so that even in older pigs, adherence to the gut and subsequent disease is markedly reduced (Frydendahl K et al., 2003). This represents a simple and effective control strategy if available.
In summary, pulling back from a laboratory diagnosis to consider the broad set of factors that influence colibacillosis in pigs can help focus control strategies more logically and productively.




