Swine AI studs have evolved in recent years from small laboratories, most of them on farm, to large professional artificial insemination studs that supply semen doses to a large number of farms. This transformation has encouraged a greater specialization in the work done in these laboratories.
Due to the implementation of post-cervical insemination, where low volume sperm doses are used (Watson and Behan, 2002, Hernández-Caravaca et al., 2012, Soriano-Úbeda et al., 2013), the control to be performed at the AI studs must be even more comprehensive. The use of post-cervical insemination has led to a reduction in the number of boars at the studs in recent years and, therefore, both the condition of the boars and the preparation of semen doses should follow a strict control. The impact of ejaculates used for post-cervical AI is 2 to 3 times higher compared to those used in cervical insemination (Mezalira et al., 2005; Hernández-Caravaca et al., 2012; Bortolozzo et al., 2015).

The main objective of the AI studs is to ensure that semen doses are in the best possible condition at the time of AI. Once the ejaculate has been collected, it must undergo several processes and controls for adequate preparation of the semen doses.The factors we consider most important include:
- Temperature control of ejaculate and semen doses
- Parameters of semen quality
- Sperm concentration
Temperature control of ejaculate and sperm doses
The drop in temperature of the sperm from its collection to the moment in which the processed semen dose is taken to storage at 15 °C must be performed progressively and carefully to avoid damaging the sperm. First, the collection cup used must be at the same temperature as the semen collected (38 °C). After collection, all the processes have to be aimed at guaranteeing a temperature control, achieving a slow and continuous reduction until reaching 15 °C, temperature at which the semen doses are stored. Dilution (semen + diluent) will be carried out at 33 °C, and once it has been packaged, the dose will remain in the laboratory until the temperature has become stable, after which time it will be introduced into the storage cabinet, so that temperature continues to fall down to 15 °C.
In order to check the temperature dropping time of a semen dose prepared at 33 °C in a laboratory with a room temperature of 24 °C, a data logger was introduced in the seminal dose (90 ml) after dilution. As shown in Figure 1, thermal stability of the dose was achieved in about 100 minutes. From that moment on, temperature drops very slowly, so in order to preserve an optimum temperature decrease, the semen doses must be introduced in the corresponding storage cabinet (15 °C).
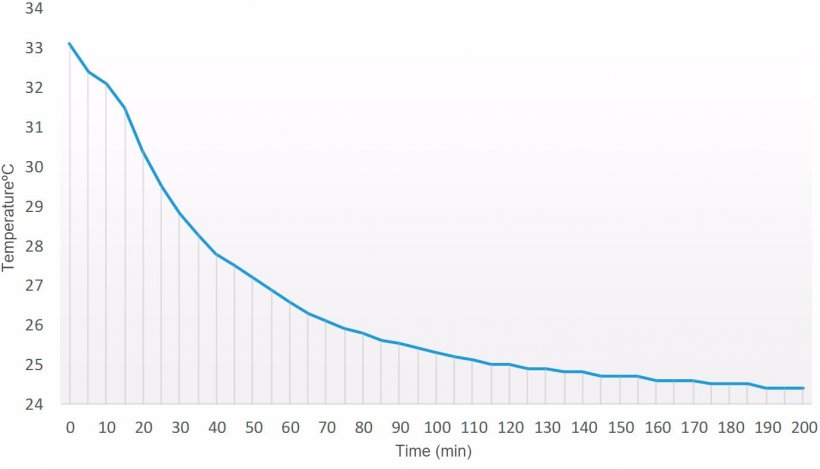
Figure 1. Temperature drop over time curve (min) of a semen dose (90 ml) prepared at 33 °C in a laboratory with a controlled room temperature of 24 °C.
Parameters of semen quality
Sperm motility, together with sperm morphology, may be the generic parameter most used at the studs to evaluate sperm quality, due to its importance during semen transport inside the female genital tract (Hernández-Caravaca Et al., 2015, García-Vázquez et al., 2015a, 2015b). The methods used to evaluate sperm motility are mainly two:
- Microscope subjective evaluation: this methodology has been, and still is, widely used in laboratories. Its aim is to provide an estimate of the percentage of motile sperm cells in a visual field under the microscope, as well as their progressive motility (usually rated on a scale of 0 to 5, where the 0 is non-progressive spermatozoa and 5 is spermatozoa with a rapid, progressive and rectilinear movement). It is a fast method and it provides an approximate estimate of sperm motility, but it is inaccurate and subjective, leaving the evaluation to the discretion of the technician (Verstegen et al., 2002).
- CASA system (computer-assisted semen analyser): this method consists in the use of a computer program that analyses the semen sample and evaluates different parameters related to motile sperm cells, such as number of motile sperm, progressive motility (%), as well as different speed parameters. This analysis system provides more objective and accurate values than the previously described method (Verstegen et al., 2002; Broekhuijse et al., 2011). However, in spite of the increasing implementation of this type of systems in porcine studs, routine checks must be carried out to ensure their accuracy. In our case, in order to validate the proper function of these systems, we chose two different laboratories where the percentage of progressive motility was measured with the same CASA system; the semen doses analysed came from the same boars, and were collected on the same day and processed with the same preparation method (Figure 2.)

It is also important to perform measurements systematically, using always the same process, and taking into account different factors such as sample temperature, dilution rate, time elapsed from the preparation of the sample to its analysis or type of chamber used (Gączarzewicz, 2015). Another factor to consider is sample volume (μl). Thus, Figure 3 shows a variation of the motility values obtained with the CASA system according to the volume of the sample used, with the highest motility correlating to the largest sample volume (28 µl.)
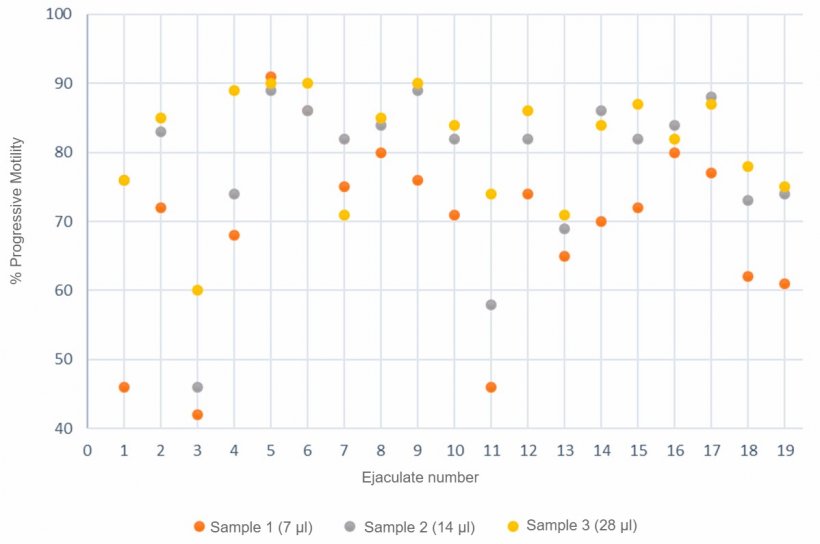
Figure 3. Progressive motility (%) analysis by computerized systems using 3 different semen sample sizes.
It is important to establish an adequate and constant analysis procedure that guarantees an adequate motility analysis. Any detail, small as it may seem, is essential, since, for example, in the case of the motility calculation, this value will be applied to correct the total concentration to be used per semen dose.









