Influenza A virus (IAV) is the cause of an economically important respiratory disease in swine around the world. As the IAV ecology has become more complex due to viral reassortment and mutation, so have the sample types and diagnostic tests available for detecting or diagnosing influenza infections in swine.
Diagnostic tests are available for direct or indirect detection of active influenza infection or previous exposure, respectively. Sample collection for direct testing should target virus replication and shedding that occurs from 1- 8 days post infection. Antibody responses may be detected from 7 – 21 days post-exposure and ideally with paired serum samples collected 2-4 weeks apart. Diagnostic samples, regardless of the type, should be chilled to 4°C and transported to a diagnostic lab using ice packs within 48 hours (Figure 1).

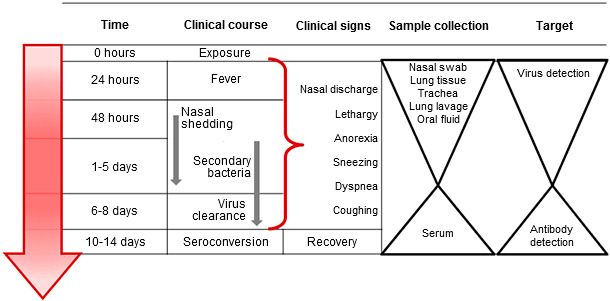
Figure 1. Targeted sample collection for direct or indirect detection and diagnosis of influenza A virus infection in swine. Sample collection for direct detection should occur during viral shedding early in the course of clinical disease. Indirect detection for influenza antibody should occur after viral clearance and target the immune response to infection.
Real-time reverse transcription-polymerase chain reaction (RT-PCR) assays are common diagnostic tests used as a screening tool for IAV infections in many laboratories on ante and postmortem samples that may include oral fluid, nasal secretions and lung tissue. RT-PCR is a rapid, sensitive and specific test based on detection of IAV genetic material, or RNA, with a quick turn-around time. In addition, RT-PCR can be used to subtype influenza infections and determine if the virus is H1N1, H1N2 or H3N2. RT-PCR results from antemortem samples should be interpreted with caution considering a positive result may be obtained from non-clinical animals in spite of the presence of the virus. Influenza sequencing of specific genes, such as the hemagglutinin, and virus isolation are also available when characterizing a particular IAV in a population.
Immunohistochemistry (IHC) is a test appropriate for diagnosing influenza infection in swine but limited to formalin-fixed tissues. A chemical-reaction creates a signal identifying influenza viral antigen that must be viewed under a microscope. An advantage of the IHC is that virus signals are associated with influenza lesions in lung tissue. However, formalin fixation and sample selection may affect the sensitivity or specificity of the test.
Antemortem diagnostic samples useful for detecting the presence of IAV in swine include oral fluids and nasal secretions. Oral fluids have become more common as a diagnostic screening tool due to the ease of collection and the ability to represent a large population. Oral fluids should be collected for approximately 20-30 minutes using cotton ropes that hang shoulder level. Avoid extraneous contamination such as feed, feces and organic material. Nasal secretions must be collected using a synthetic swab (rayon or dacron) with a plastic shaft and transport fluid appropriate for viruses to prevent the swab from desiccation.
Oral fluids may be difficult to collect from neonatal piglets but are appropriate for weaned, grower, finisher and adult swine. A disadvantage of oral fluids is the sample type contains inhibitors that may affect detection by RT-PCR and the presence of low quantities of virus may preclude sequencing or virus isolation. Nasal swabs are typically more successful for sequencing and isolation of influenza due to larger quantities of virus in the sample but require sample collection from individual swine of sufficient number to represent the population during the acute phase of infection.
A diagnosis of IAV clinical disease in swine requires post-mortem examination and samples that should include formalin-fixed and fresh lung tissue, trachea or lung lavage fluid. Influenza-infected pulmonary tissues typically display purple-red, multifocal consolidation of the cranioventral portions of the lungs. A pathologist will view necrosis of the epithelium in the airway (bronchi or bronchioles) of affected lungs. Correlating the clinical signs, gross and microscopic lesions with a positive ancillary test, such as RT-PCR or IHC, confirms a diagnosis of influenza infection and clinical disease (Figure 2).
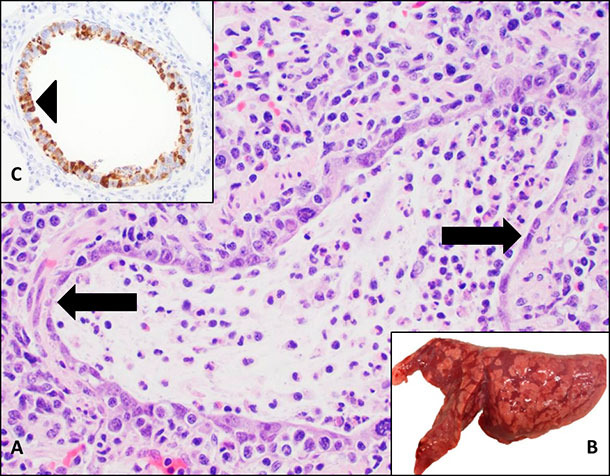
Figure 2. Influenza A virus is an important cause of respiratory disease in swine. A. Microscopic lesions consistent with influenza infection include necrosis of the bronchiolar epithelium (arrow) where the virus replicates. B. Gross lung lesions manifest as purple-red consolidation demonstrating a checker-board pattern. C. Influenza A virus can be detected by immunohistochemistry (IHC) as brown signals associated with necrosis of the respiratory epithelium (arrowhead).

Serum is an antemortem sample used to detect IAV antibody in swine. A blocking ELISA assay directed against the conserved influenza nucleoprotein is a screening tool to confirm virus exposure, vaccination or in the case of neonatal or weaned pigs, maternal antibody. However, the IAV ELISA is not useful to predict protection against infection. Functional antibody assays, such as hemagglutination inhibition (HI) or serum virus neutralization (SVN), may help predict cross-protection when using the appropriate anti-sera and virus antigen. The use of influenza vaccines and active endemic infections in many regions may limit the benefit of serological testing for influenza. Interpret serological results within the context of the specific characteristics of the herd and a thorough history.
Collectively, when diagnosing influenza infection or clinical disease in swine, consider the diagnostic question and necessary tests to achieve the appropriate outcome. Consult a veterinarians and/or diagnostic laboratory and use their specific protocols for sample collection and testing to maximize the benefits of diagnostic tests for IAV in swine.



