Assays available
Polymerase chain reaction (PCR)
- Detects presence of specific sequence of viral nucleic acid (RNA)
- Sample types: intestinal tissues, feces, rectal swabs, oral fluids, etc.
- Pros:
- Very high sensitivity (can detect small amounts of virus)
- Early detection - acute cases should be positive
- Many different sample types can be used (intestinal tissue, feces, rectal swabs, oral fluids, etc.)
- Moderate cost
- Can often do pooling of 10 fecal samples/swabs or intestinal tissue samples to lower cost while minimizing loss of sensitivity
- Often do not pool oral fluids due expected higher Ct values (lower virus concentrations) which can result in significant loss of sensitivity
- Cons:
- Sequencing needed to differentiate vaccine virus vs. wildtype infection
- Environmental contamination may generate many positive results - Large amounts of virus are shed by infected pigs
Enzyme-linked immunosorbent assay (ELISA)

- Detects presence of antibodies
- Sample types: serum, oral fluids
- Pros:
- Animals remain positive for several weeks especially IgA in oral fluids
- Can be used in chronic cases
- Cons:
- Can have differences in detection between different assays
- Takes 7 to 14 days for animals to become seropositive
- Unable to differentiate maternal antibodies vs exposure
- Unable to differentiate vaccine vs. wildtype infection
- Best to have an ELISA that detects IgA levels especially for oral fluids
Immunohistochemistry (IHC)
- Detects presence of viral antigen
- Sample types: intestinal tissues
- Pros:
- Detects virus at site of lesion (good proof of causation)
- Can identify low vs moderate vs high amounts of virus present
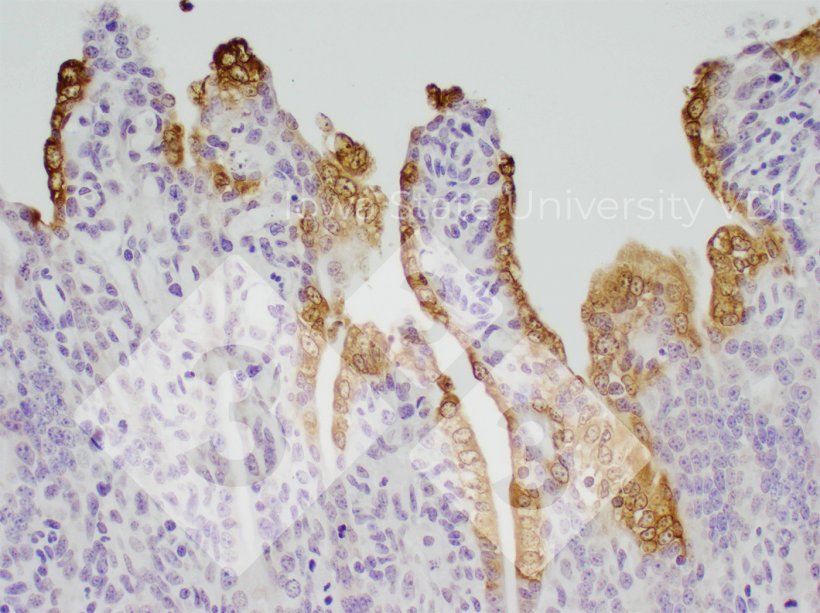
- Cons:
- Correct tissue sample must be submitted
- Requires significantly more virus to be present than PCR
- Only evaluating a small tissues sample
- In piglets the intestinal villi are killed quickly resulting in dramatic changes in amount of virus present (staining) in just 2 days
Indirect fluorescent antibody (IFA)
- Detects presence of antibodies
- Sample types: serum
- Pros:
- High sensitivity for detection
- Cons:
- Not feasible for large number of samples
- Results impacted by virus isolate used for assay
- Reliability is highly dependent on technician skills and vary significantly between labs
Result interpretation
PCR
- Positive – virus is present. Recent vaccination with a modified live virus can result in positive PCR results. Very large amounts of virus (very low Ct values; single digit) are shed after infection, especially in young pigs. Shedding and environmental contamination can occur for a long period of time.
Table 1. Large amounts of virus particles are shed in feces of infected piglets (single digit Ct values in PCR) resulting in large amounts of environmental contamination.
| PED PCR Ct versus number of genomic copies | |
|---|---|
| 42 | 1 |
| 39 | 10 |
| 36 | 100 |
| 32 | 1,000 |
| 29 | 10,000 |
| 26 | 100,000 |
| 22 | 1,000,000 |
| 19 | 10,000,000 |
| 16 | 100,000,000 |
| 12 | 1,000,000,000 |
| 9 | 10,000,000,000 |
| 6 | 100,000,000,000 |
- Negative – Negative or virus could have been missed if testing occurs late after infection
ELISA
- Positive – Maternal antibodies or past exposure (usually > 7-14 days post exposure) to vaccine or wildtype virus
- Negative – Negative, infection too early to detect (usually must be at least 7-14 days post exposure), or too long after infection (especially if measuring serum IgG)
IHC
- Positive – Virus is present at site of lesion
- Negative – Negative or infection is too late to detect virus
IFA
- Positive – Maternal antibodies or past exposure (> 7-14 days post exposure) to vaccine or wildtype virus
- Negative – Negative to vaccine or wildtype virus or infection too early to detect (must be at least 7-10 days post exposure)
Scenarios
Piglet diarrhea with very high mortality in farrowing house
- Intestines from 2-5 piglets for histology and IHC testing
- Intestines or fecal content/swabs from 5 piglets and test as 1 pool sample via PCR
Gilts with diarrhea +/- vomiting
- Collect oral fluids from 2 to 4 different pens and test individually via PCR. Do not pool samples for testing.
- Collect 10 to 15 rectal swabs from pigs with clinical signs or random sampling and test via PCR. Can pool in groups of 5 for PCR testing.
Testing gilts in isolation post exposure to live PED virus from farm before bringing into herd
- No testing is recommended as environmental contamination will be high and gilts will test positive via feces or oral fluids for several weeks even after they have stopped shedding virus and are safe to introduce into the breeding herd
Growing pig with acute diarrhea post weaning
- Collect oral fluids from 2 to 4 different pens and test individually via PCR. Do not pool samples for testing.
- Collect 10 to 15 rectal swabs from pigs with clinical signs or random sampling and test via PCR. Can pool in groups of 5 for PCR testing.