Assays available
Culture
- Isolation on live organism
- Sample types: feces
- Pros:
- Relatively inexpensive
- Presence of strong beta-hemolytic Brachyspira spp. is associated with virulence
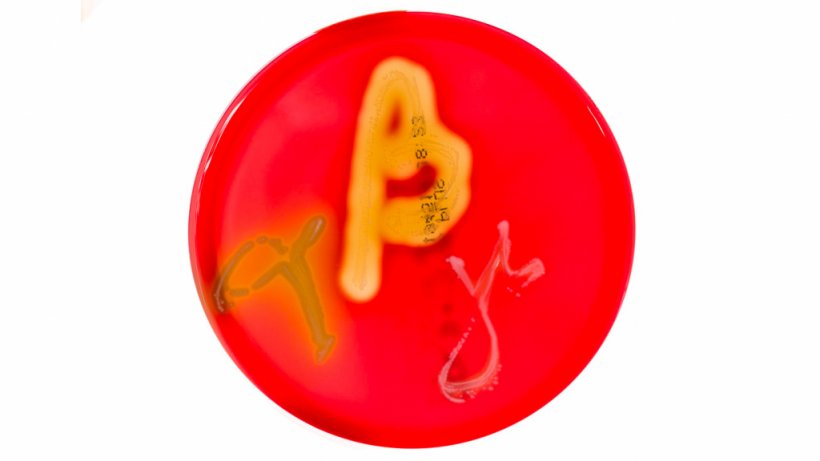

Table 1: Link between beta-hemolysis in Brachyspira spp. and disease
| Brachyspira spp. | Beta-hemolysis | Disease |
|---|---|---|
| B. hyodysenteriae | Strong | Swine dysentery lesions |
| B. hampsonii | Strong | Swine dysentery lesions |
| B. suanatina | Strong | Swine dysentery lesions |
| B. pilosicoli | Weak | Spirochetal colitis |
| B. murdochii | Weak | Spirochetal colitis |
| B. intermedia | Weak | Spirochetal colitis |
| B. innocens | Weak | Non-pathogenic |
- Cons:
- Less sensitive than PCR assay
- Slow growing (up to 10 days)
- Require anaerobic culture
- Some less virulent isolates can be found in herds with no clinical signs or lesions
Antimicrobial susceptibility
- Tests in vitro: ability of live organism to grow under specific concentrations of different antimicrobials.
- Sample types: feces
- Pros:
- Identification of susceptibility or resistance of specific isolate to common antimicrobials
- Cons:
- Requires a bacterial isolate
- In vitro testing results may be slightly different than in vivo results
- Some specific antimicrobials may not be tested or require separate, special testing
- Moderate cost
- Takes 7 days to get results
Histopathology
- Evaluates the presence of tissue lesions which can support the presence of disease
- Sample types: tissue
- Pros:
- Histopathology lesions can be highly suggestive if many spirochete bacteria near intestinal lesions are found
- Can detect mild versions of the disease
- Cons:
- Need formalin-fixed intestinal tissues from recently deceased animal
- Lesions are similar to several other diseases
Impression smear
- Detects presence of bacteria
- Sample types: large intestine tissue, feces
- Pros:
- Easy to do
- Non-specific staining
- Dark-field microscopy
- Presumptive diagnosis can be made by detecting a large number of spirochetes

Figure 2. Graphic demonstrating the spirochetal shape of Brachyspira spp. Source: Y_tambe. https://creativecommons.org/licenses/by-sa/3.0/deed.en - Can identify low vs moderate vs high amounts of bacteria present
- Easy to do
- Cons:
- Requires significantly more bacteria to be present than culture or PCR
- Unable to assess hemolytic capability of isolates and thus cannot differentiate non-pathogenic from spirochetal colitis from swine dysentery
Polymerase chain reaction (PCR)
- Detects presence of specific sequence of bacterial nucleic acid (DNA)
- Sample types: intestinal tissues, fecal swabs, feces
- Pros:
- High sensitivity
- PCR quantification is associated with presence of lesions
- Moderate cost
- Can often do pooling of feces or tissue samples to lower cost while minimizing loss of sensitivity (especially regarding clinical relevance).
- Cons:
- High sensitivity – confirms presence of bacteria but not disease
- Proper primers are needed to detect and differentiate between Brachyspira spp
Enzyme-linked immunosorbent assay (ELISA)
- Detects presence of antibodies
- Sample types: serum
- Pros:
- Can detect infection at a herd level
- Antibodies can last several months
- Serum IgG levels correlate with duration of clinical signs
- Cons:
- Assays not readily available
- Low sensitivity and low specificity
- Used for herd detection and not individual animal diagnosis
- Specific antibodies detected and the timing of detection may vary between the different assays
- Often antigens used can be specific to particular species of Brachyspira
- No correlations with antibody levels and protection
- Cross protection between Brachyspira spp is unknown
Result interpretation
Culture
- Positive: Bacteria present
- Strong beta-hemolysis – swine dysentery
- Weak hemolysis – spirochetal colitis or non-pathogenic
- Negative: Negative or animal possibly previously treated with antibiotics or too late into infection
Antimicrobial susceptibility
- Susceptible: possible good choice for treatment if antimicrobial can reach target tissue
- Resistant: select different antimicrobial
- MIC: MICs are done to ensure the antimicrobial selected achieves the listed MIC value in the target organ
Histopathology
- Positive: Spirochetes found near lesions are presumptive diagnosis
- Negative: No intestinal lesions
Impression smear
- Amount of spirochetes present:
- High: highly suggestive of disease
- Moderate: variable interpretation
- Low: questionable value (could be non-pathogenic or chronic condition)
- Not present: Animal possibly previously treated with antibiotics or not significant contributor
PCR
- Positive: confirms bacterial presence
- Negative: Negative or bacteria could have been missed if testing occurs on animals treated with antibiotics or too late after infection
ELISA
- Positive: Past exposure (>2-4 weeks) to vaccine or wildtype bacteria
- Negative:
- Negative to vaccine or wildtype bacteria
- Infection too late to detect
- Antibiotic use may suppress bacterial growth and lower immune response
Scenarios
Growing or finishing pigs with scours (acute)

- Collect fecal samples from 10 or more untreated scouring pigs and submit for culture and PCR testing in pools of 5.
- Necropsy of 1-3 recently dead or euthanize scouring pigs. Grossly evaluating large intestine (especially cecum and spiral colon) place in formalin solution for histopathology.
Chronic history of swine dysentery diagnosis but no clinical signs noted
- Collect feces samples from 4-6 pens and submit for PCR testing
- Collect samples from 15-20 pigs at 14 and 18 weeks of age
- Two approaches to collecting:
- Cross-section – collect from different age groups at one time (quicker to obtain results)
- Longitudinal – collect from same pigs over time (results are more accurate)
- Serum samples test via ELISA
- Two approaches to collecting: