App may cause three types of infections:
- acute (high or intermediate mortality level)
- chronic (low mortality, few and low specific clinical signs, reduced growth rates and/or lesions at slaughter)
- subclinical (no clinical signs, no lesions at slaughter).

The diagnosis of the acute form is usually not complicated
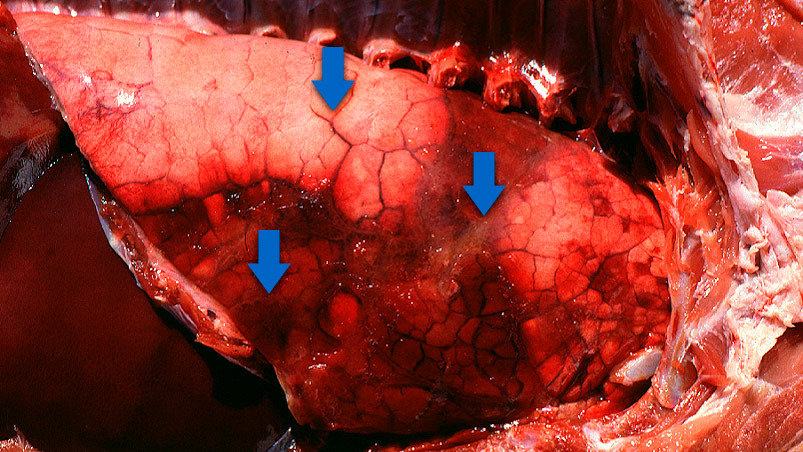
- Affected animals usually present lung lesions which are quite pathognomonic of App-affected animals. Samples should be taken from affected lungs and sent to the laboratory.
- Any diagnostic laboratory with experience on swine bacterial isolation would be able to correctly isolate and identify App. However, in certain European countries (for ex. Spain, Belgium, France), atypical App strains (called biotype II isolates) are present and this may be challenging for some laboratories.
- Identification of the serotype may be important: in an affected herd, this information will indicate the level of risk. High virulent serotypes are primary pathogens; intermediate virulent serotypes usually cause mortality in high-health status herds, or in the presence of co-infections and/or other predisposing factors. Knowing the serotype is also useful to identify which commercial bacterin may be used in a given herd. Finally, there is a need to know the distribution of virulent serotypes in a given area to evaluate the risk of sub-clinically infected herds (see below). Serotyping is now done by PCR and may be available for any diagnostic laboratory.
The diagnosis of the chronic form is somehow more difficult
- Pleurisy lesions observed at slaughter are somehow characteristic but not necessarily pathognomonic.
- Detection of App in such lesions, especially when they are relatively old, is very difficult and false negative results are usually obtained.
- Very often co-infections (such as Mycoplasma hyopneumoniae) are also present and complicate the diagnosis.
- If some clinical signs are present, a sero-profile using ApxIV toxin-ELISA (see below) may be done, first at the presence of clinical signs and then 21-28 days after, to confirm a clear sero-conversion.
- In the absence of clinical signs, lesions at slaughter along with clear and high ApxIV toxin-ELISA positive results might be an indication of App infection: between 25 and 30 serum samples should be tested. If positive, cross-sectional serum samples may be taken to confirm: 10 samples once a week from 10 to 20 week of age. A herd susceptible to be affected by App would be those with clear positive serological results that increased at a given time.
- In the absence of clinical signs, lesions at slaughter with the presence of LPS-based ELISA positive results (see below) for a virulent serotype (in a given country) is an indication of App infection. Serum samples at slaughter may be taken (between 25 and 30 serum samples).
The diagnosis of sub-clinically infected herds must be done through serology
Due to the absence of clinical signs and lesions it is not easy to diagnose sub-clinical infections due to App. Usually, animals are not affected (feed conversion, growth rate, etc.). This becomes an important issue for breeding herds: subclinical infected animals may mean "carrier animals" that may transmit App from tonsils to other more susceptible animals. A virulent strain may not induce disease in one herd but may cause high mortality in another more susceptible herd. Even within one herd, changes on herd management, environmental conditions or the arrival of co-infections may transform sub-clinical infection with a virulent strain into an outbreak of pleuropneumonia. Serology is an important tool to diagnose sub-clinically infected herds. Two questions arise:
a) Which test should be used?
Two types of ELISA tests are commercially available: App-specific Apxiv test (detecting all strains of App without distinction of serotype) and LPS-based serotype specific test (see Table 1).
Table 1: Use of commercially available ELISA test to detect antibodies against App
| ApxIV App-specific ELISA | LPS serotype/serogroup-specific ELISA | |
| Commercial herds | Not useful (most herds give positive results) | Useful only if virulent serotypes are tested |
| High health status herds (confirmed as App-free) |
Useful | Not useful (testing all individual serotypes is too expensive) |
| Sensitivity (after experimental infections) | +++ | ++++ |
| Specificity (experimental infections with other bacterial species) | ++++ | +++ |
| Routine use for the evaluation of breeders in several countries | No | Yes |
The Apxiv test will be positive in any herd infected by App (virulent or non-virulent strains). More than 70% of commercial herds will be positive with this test in most countries. LPS-based tests should be oriented to the most important and virulent serotypes, which will give you the level of risk of a given herd. There is a need to know the distribution of virulent serotypes in a given country: strains isolated from diseased animals must be serotyped to make this information available.
b) How many samples and which category of animals should be taken to diagnose an infected herd? How often a herd should be tested to be classified as “non-infected herd”?
We recommend around 30 serum samples at the end of the finishing period; in batch farrowing system different batches should be tested to avoid false negative results. To certify a herd as "free", there is no universal rule, but it is recommended to repeat the testing at least 2-3 times a year.
Finally, for breeding herds, inconclusive serological results may complicate the diagnosis. In such cases, App detection from tonsils through the use of PCR of sero-positive animals may be done. If the herd is positive for different virulent and non-virulent serotypes, a serotype-specific (for the virulent serotype detected by serology) PCR must be used. For herds supposed to be App-free, an App-specific PCR (for all serotypes) can also be used. These tests must be done by experienced laboratories; however, false negative or positive results are sometimes observed.








