Over the last years there has been an increased interest in Mycoplasma hyorhinis as a cause of polyserositis and arthritis in postweaning pigs. It is presumed sows transmit the bacterium to their offspring; however, there is limited knowledge on the epidemiology and ecology of this agent. Essential information, such as timing of colonization, postweaning transmission dynamics and role of the sow in disease spread is lacking.
In the studies summarized here modern diagnostic tests (i.e. qPCR) and collection techniques (i.e. oral fluids and nasal swabs) were utilized to expand the understanding of the epidemiology of this microorganism in today’s modern swine industry.

Study 1. Estimation of the nasal colonization prevalence of M. hyorhinis in pig farms with poliserositis
Three 6000 farrow-to-wean herds, designated as A, B and C, and their nurseries were selected. Although all three herds had history of M. hyorhinis disease, only herds A and B were experiencing high nursery mortality due to polyserositis at the time of sampling. The sampling of each herd included the collection of nasal swabs from 60 sows, 60 piglets in each group of 1, 7, 14 and 21 days of age as well as 30 pigs in each group of 28, 35, 42, 49, 56, 63, 70 and 77 days of age. Additionally, since M. hyorhinis can be detected in the oropharyngeal surface, oral fluid samples were collected from one pen per age throughout the nursery. In order to investigate the role of M. hyorhinis in polyserositis cases tissue samples were collected from ten clinically affected and ten clinically healthy pigs necropsied at the age of the peak of mortality in the nursery. Samples were tested for M. hyorhinis by a qPCR developed in our laboratory.
Study Findings
We found that M. hyorhinis was detected in the nasal cavity of 5/60 sows in herd A, 3/60 in herd B and none in herd C. In herd A and B where clinical cases of M. hyorhinis were present, the colonization prevalence in suckling piglets was low (avg=8%) and high in post-weaning pigs (avg=98%). In contrast, in herd C where M. hyorhinis clinical cases were absent, colonization in pigs was very low until the last week in the nursery. A total of 7/8 oral fluids collected from post-weaning pigs tested M. hyorhinis positive in herd A and B, while 1/8 tested positive in herd C. Polyserositis was not observed in any of the healthy animals from all three herds neither in the diseased pigs from herd three. However, in herds A and B polyserositis was observed in 9/10 and 4/10 diseased pigs respectively (Figure 1). M. hyorhinis was detected by PCR in the pericardium of 8/10 diseased pigs in herd A and 3/10 in herd B. Isolation of M. hyorhinis from the pericardium was achieved only in herds A and B. In herd C, M. hyorhinis was not detected by PCR in any of the necropsied pigs.
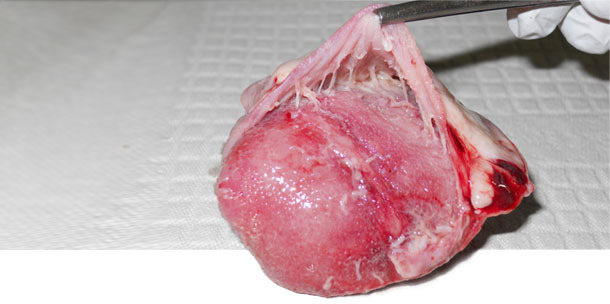
Figure 1. Heart. Pericarditis observed with M. hyorhinis systemic disease.
Study 2. Prospective longitudinal study of M. hyorhinis colonization in two commercial farms
Two breeding herds, A and B, were selected based on history of polyserositis in the pig flow and no use of M. hyorhinis vaccine. In each herd, a longitudinal sampling of pigs at different ages was performed. A total of 50 young sows (p1 and p2) and 50 older sows (p3 and older) were randomly selected and tested for M. hyorhinis by nasal swab qPCR and M. hyorhinis antibodies in serum by ELISA (Figure 2).
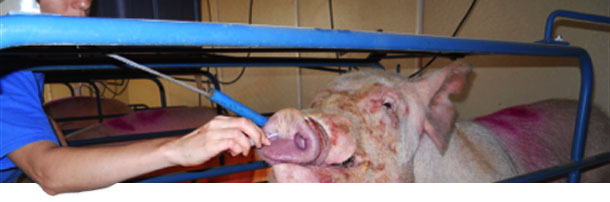
Figure 2. Collection of nasal swabs from sows for the detection of M. hyorhinis by qPCR
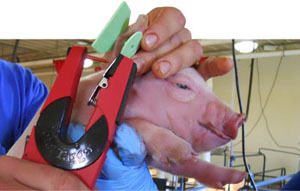
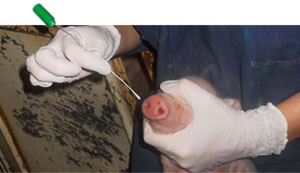
One piglet per litter was randomly selected and ear tagged from each sow, for a total of 100 piglets. A nasal swab and a serum sample was collected from each pig at birth, weaning and 10 days post-weaning (Figure 3-4). Two more sample collections were performed in the nursery and finishing stage during the peak of polyserositis/ arthritis/ pneumonia. In order to confirm involvement of M. hyorhinis in polyserositis cases a total of twelve pigs were euthanized and necropsied (10 clinically diseased and 2 clinically healthy) during these two sampling points. Oral fluids were also collected at each post-weaning sampling point for a total of 56 samples. In summary, a total of 1050 nasal swabs were collected from 216 sows and 216 pigs, at 10 different collection times.
 |
 |
|
Figure 3. Ear tagging of piglets prior to sample collection |
Figure 4. Collection of nasal swabs from piglets shortly after birth |
Study findings

M. hyorhinis was detected by qPCR in the nasal cavity of 5/107 sows in herd A, 2/110 in herd B. Four of the positive sows were parity 1-2 while 3 were parity 3. The colonization prevalence in suckling piglets was low in both herds (avg=1.7%) and high in post-weaning pigs (avg=85%). Most pigs became colonized soon after placement in the nursery and remained positive throughout the nursery and finishing stage. In both herds, antibody titer results revealed that piglets had antibodies (maternal antibodies) against M. hyorhinis shortly after birth. These antibodies started to decay soon after weaning. Approximately at 94 and 64 days of age antibody titers started to rise in Herd A and B, respectively. It is presumed that this rise was due to an active immune response. In Herd A, a total of 25/31 oral fluids were positive. Furthermore, detection of M. hyorhinis in oral fluids was consistent with detection in nasal swabs. In herd A, M. hyorhinis was detected in pericardium (12/18) and in joints (10/18) of diseased pigs. Similarly, in herd B, M. hyorhinis was detected in pericardium (5/10) and in joints (4/10) of diseased pigs. This contrasted with an absence of M. hyorhinis in the same tissues of healthy cohorts. All necropsied pigs were M. hyorhinis PCR positive in the nasal cavity.
Final Considerations
M. hyorhinis is a common inhabitant of the nasal cavity of swine. However, not all pigs are colonized in a population and not all get colonized at the same time. We presume that the very few colonized piglets at weaning are responsible for post-weaning transmission.
The use of oral fluids for detection of M. hyorhinis appears to be a useful tool to track the infection dynamics in a herd and allows for better timing of antibiotic treatment or vaccination. However, additional validation for the detection of M. hyorhinis in oral fluids is required.
Knowledge of the dynamics of infection within the herd will allow implementation of better control strategies in affected herds.



