Farm introduction
The present study reports on a breeding stock of a Greek farrow-to-finish commercial pig farm. The capacity of the farm was 650 sows under production (commercial hybrids of Large White × Landrace) and a grandparent nucleus of 35 sows was kept for the production of F1 gilts. Weaned sows and gilts were housed in individual stalls for 30-35 days and they were artificial inseminated (AI) twice with fresh semen from the same boar. At 30-35 days of gestation, sows were moved to group housing pens (10 animals/group). The farm’s vaccination scheme of breeding stock and weaners is shown in Table 1. Antiparasitic preventive programme included a single ivermectin injection 14 days prior to farrowing for sows and twice a year for boars.

The farm was PRRSV seropositive and one year before the study an outbreak of PRRSV infection in breeding stock and weaning to finishing stage was noticed [PCR positive breeding stock (30%), weaners (90%) and growers/finishers (35%)].
Table 1. Vaccination scheme of breeding stock and weaners
| Vaccination against diseases | Scheme |
| Gilts | |
| Aujeszky’s Disease Virus | 90th + 120th day of age |
| Parvovirus + Erysipelas | 150th + 180th day of age |
| Atrophic Rhinitis | 150th + 180th day of age |
| PRRSV | 180th + 210th day of age |
| Escherichia coli + Clostridium perfringens | 160th + 190th day of age |
| Sows | |
| Aujeszky’s Disease Virus | 4 weeks before farrowing |
| Parvovirus + Erysipelas | 2 weeks after farrowing |
| Atrophic Rhinitis | 3 weeks before farrowing |
| PRRSV | 60th day of gestation + 6th day of lactation |
| Escherichia coli + Clostridium perfringens | 2 weeks before farrowing |
| Boars | |
| Aujeszky’s Disease Virus | 3 times per year |
| Parvovirus + Erysipelas | 3 times per year |
| Atrophic Rhinitis | 3 times per year |
| Piglets | |
| Mycoplasma hyopneumoniae | 7th + 21st day of age |
| Porcine Circovirus type 2 | at weaning day |
Case study
An erythema multiforme (EM) was noticed in the majority (90%) of breeding stock, associated with respiratory problems and without mortality. Since January 2014 up to our clinical examination on May 2014, the sows once moved to the group housing pens (~ 30-35 days of gestation) developed EM, characterized by raised skin areas of red colour on all over the body, especially on the neck, the face, around the eyes and ears (Figures 1-3). In addition, depression, anorexia, high fever (40-41,5oC) for 1-3 days, posture difficulties and respiratory signs (moderate breathing difficulties, eye and nasal discharge, accompanied with mucus and blood / Figures 4-5) were noticed in the diseased sows. The above symptoms were not detected in lactating and recently weaned sows, or in the building of newly introduced gilts that were not yet inseminated. This clinical signs were obvious and remained for several weeks in the majority (~ 90 %) of pregnant sows once mixed in the group housing pens, as well as in sows/gilts of grandparent nucleus. Moreover, 10 to 15% of inseminated sows and gilts returned to oestrus shortly after mixing them in the group housing system (Table 2).
Table 2. Farm reproductive parameters in 2014
| Reproductive parameters | Months | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Farrowing rate (%) | 72 | 68.7 | 71.4 | 73.8 | 74.9 | 78.1 | 77.3 | 67.4 | 76.3 | 85.0 | 92.5 | 94.2 |
| Abortions (%) | 1.9 | 2.2 | 2.4 | 2.1 | 2.0 | 1.8 | 1.6 | 1.5 | 1.1 | 0.5 | 0.4 | 0.2 |
| Returns to oestrus (%) | 26.0 | 29.0 | 26.0 | 24.0 | 23.0 | 20.0 | 21.0 | 31.0 | 22.5 | 14.4 | 7.0 | 5.5 |
| Failure to farrow (%) | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Pigs born alive/litter | 13.2 | 13.2 | 13.1 | 13.2 | 13.2 | 13.1 | 13.3 | 13.1 | 13.2 | 12.9 | 13.1 | 13.1 |
| Pigs weaned/sow | 10.7 | 10.9 | 10.8 | 10.8 | 10.9 | 11.0 | 11.1 | 11.1 | 11.2 | 11.4 | 11.6 | 11.8 |
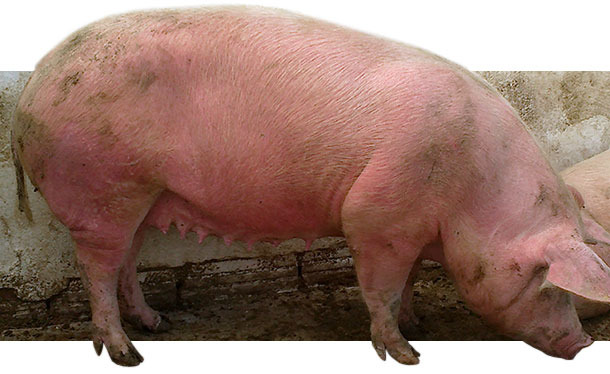

Figures 1-2. Diseased sows in group housing pen with EM, characterized by red, raised skin areas that appeared all over the body.
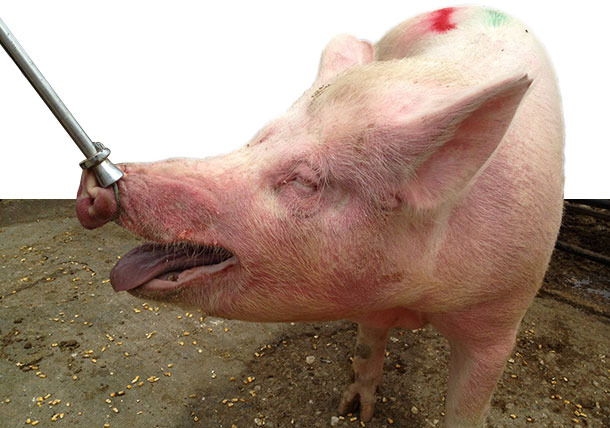
Figure 3. Diseased sow in group housing pen with EM, characterized by red, raised skin areas on the neck and the face (especially around the eyes and ears).

Figures 4-5. Diseased sows with EM, accompanied with depression, anorexia, fever, moderate breathing difficulties, eye and nasal discharge with mucus and blood.
Sampling and diagnostic examinations
On May 2014 blood samples from 7 diseased sows as well as nasal and vaginal swabs from other 4 diseased sows were collected (sampled sows at 30-45 days of gestation from parity 1 to 5). In addition, on August 2014 nasal samples from 7 diseased sows were collected.
Serum blood samples were examined by: a) Quantitative PCR (qPCR) for PRRSV (type 1-PRRSV EU and type 2-PRRSV US) and for PCV2, and b) ELISA for Actinobacillus pleuropneumoniae (App) antibodies (Abs), Erysipelothrix rhusiopathiae (Ery) Abs, PRRSV Abs, ADV gE Abs, African Swine Fever Virus (ASFV) Abs, Classical Swine Fever Virus (CSFV) Abs, and Leptospira spp. Abs. Nasal swabs were examined by PCR for apx-IV gen of App, Bordetella bronchiseptica, Haemophius parasuis, Pasteurella multocida, Streptococcus suis, Porcine Cytomegalovirus (PCMV, Inclusion Body Rhinitis) and Swine Influenza Virus (SIV) (H1N1, H3N2, H1N2).
In addition, 2 blood samples (with anticoagulant, strict aseptic technique and sterile equipment) from diseased sows were collected for blood culture. Moreover, haematological and biochemical analyses were performed of the blood samples. At slaughterhouse, samples from 7 diseased sows (skin, liver, kidney, lung) for gross and microscopic examinations, were collected.
Moreover, samples of the complete gestation and lactation feed were examined for mycotoxines [Aflatoxines (B1, B2, G1, G2), Deoxynivalenol (DΟN), Acetyldeoxynivalenol (acetyl-DΟN), Nivalenol, Zearalenone (ΖEΝ)], using HPLC (Varian 9010 & 9050) method.
Laboratory results
The results of ELISA and PCR exams in serum blood samples are shown in Table 3. All sows were seropositive for App and PRRSV, but seronegative for ASFV, CSFV, ADV and Leptospira spp. In addition, no viraemia for PRRSV and PCV2 was detected. The results of nasal swabs are presented in Table 4. B. bronchiseptica, P. multocida and PCMv were not detected in any sample. S. suis was detected in all samples, one sample (No 11) was positive for App, H. parasuis and S. suis. One sample was positive for SIV on May 2014 sampling.
Table 3. Results of analyses of serum samples from seven sows showing skin lesions and respiratory symptoms
| Pathogens | Test | No of positive pigs / No of tested | |
| May 2014 | August 2014 | ||
| Actinobacillus pleuropneumoniae | APX-IV ELISA | 7/7 | 4/7 |
| Erysipelas | ELISA | 0/7 | 0/7 |
| African swine fever virus | ELISA | 0/7 | 0/7 |
| Classical swine fever virus | ELISA | 0/7 | 0/7 |
| Porcine Circovirus type 2 | qPCR | 0/7 | 0/7 |
| PRRSV* | ELISA | 7/7 | 7/7 |
| PRRSV Type 1 (EU) | PCR | 0/7 | 0/7 |
| PRRSV Type 2 (US) | PCR | 0/7 | 0/7 |
| Aujeszky's disease virus | gE Abs ELISA** | 0/7 | 0/7 |
| Leptospira spp. | ELISA | 0/7 | 0/7 |
*PRRSV antibody ELISA Results: < 0.4 negative; 0.4-0.99 positive 1; 1.0-1.49 positive 2; 1.5-1.99 positive 3; 2.0-2.49 positive 4; 2.5-2.99 positive 5; ≥ 3.0 positive 6
** positive: field virus infected (antibodies against AK gE) - negative: not infected with field virus (no antibodies against AK gE).
***L. Pomona, L. tarassovi, L. canicola, L. grippothyphosa, L. Bratislava: all samples were < 1:100
Table 4. Results of PCR exams in nasal swabs from diseased-sows
| No of positive pigs / No of tested | Pathogens | ||||||
| App | B. bronchiseptica | H. parasuis | P. multocida | PCMv | S. suis | SIV | |
| May 2014 | 1/4 | 0/4 | 1/4 | 0/4 | 0/4 | 4/4 | 1/4 (H1N1) |
| August 2014 | 1/7 | 0/7 | 6/7 | 0/7 | 0/7 | 7/7 | 0/7 |
The examination of vaginal swabs revealed E. coli and Streptococcus spp. infections in all samples. Streptococcus spp and Actinobacillus spp were isolated from blood culture, while the antibiogram revealed that they were sensitive to: penicillin, ampicillin and amoxycillin + clavulanic acid combination.
Haematological and biochemical analyses indicated moderate leukocytosis with atypical lymphocytes and lymphopenia (possibly secondary to the depletion of CD4 lymphocytes), mild anemia and thrombocytopenia in one case. An eosinophil count greater than 3000/μL was also seen in two cases. A severely elevated total white blood cell counts in two cases was consistent with infection. The histopathological examination of the skin revealed increased vascularization of the superficial and middle dermis mainly (Figure 6). No remarkable lesions were found in the other examined organs.
No reveal mycotoxins at detectable levels were noticed in the gestation and lactation feed.
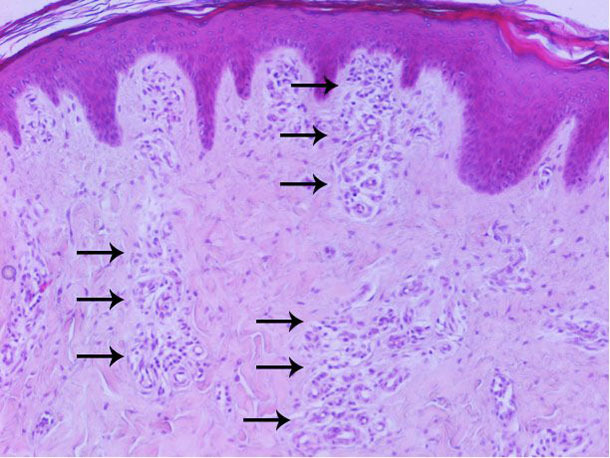
Figure 6. Histopathological examination of the skin: increased vascularization of the superficial and middle dermis mainly.
Measures taken
On May 2014, the treatment against E. coli and Streptococcus spp. infections of reproductive system included injectable amoxycillin + clavulanic acid was applied in all sows at the day of weaning, while the 1st week of the introduction of sows/gilts in the group housing pen 400 ppm of amoxycillin were administrated in the feed. In addition, acetylsalicylic acid powder was given as top dressing for 3 days in all diseased sows. Finally, to reduce agression among sows, a commercial product (based on herbs) with bitter taste was used locally on the skin around the neck and upper back.
On August 2014, vaccination against SIV and H. parasuis was included in the routine vaccination programme of sows/gilts, and against App in the routine vaccination programme of replacement gilts. Two months after the start of vaccinations, the clinical signs (EM, respiratory signs etc) decreased dramatically.
Case evolution / Conclusions
Stress impairs immune functions, so infections are more likely to affect pigs under stressful conditions of group housing systems. In addition, stress may cause a subclinical infection to be activated. In the current clinical case, it is possible that inseminated sows/gilts were subjected “stress” at a time when their immunity status was low, making them more susceptible to become infected and develop disease. It is known that crowding and mixing may cause stress, negatively influencing the immune function. As EM is a hypersensitivity reaction usually triggered by infections, or illness, the possible explanation of the clinical manifestation of EM in this case study could be fever (due to S. suis, H. parasuis and App infections) and possibly the stress (due to mixing and fighting). The possibility of SIV infection to be included in the etiology is low, as it had not spread to other sows and normally it should not only have occurred in sows after mixing them in the group housing.
Nowdays, after the initial control of clinical signs and the application of booster vaccinations against SIV and H. parasuis, we usually notice sporadic and extremely decreased cases in number and severity of signs. These sporadic cases is remained mainly in gilts, after their AI and mixing in groups (>30-35th day of gestation). The treatment protocol includes injectable amoxycillin + clavulanic acid and meloxicam/48h for two times. Alternatively, injectable amoxycillin + clavulanic acid/48h for two times and top dressing acetylsalicylic acid powder for 3 days is proposed.
In conclusion, stress under group housing conditions could be a possible trigger factor for this clinical case, as a subclinical infection or an interaction of different respiratory pathogens seems to have been activated, affecting negatively the health status and performance of the breeding stock.








