Epidemiology of post-weaning colibacillosis and oedema disease
These are common global infection among farmed pigs – these strains of E. coli appear to be "embedded" in most pig farms, so elimination is not a current option. Vaccination of sows or gilts with ETEC vaccines has no effect on post-weaning E. coli infections. Affected pigs are often derived from gilt litters and after weaning, the loss of sow milk and IgA assists strains of resident E. coli to proliferate.

The common on-farm risk factors for the occurrence of post-weaning E. coli disease are:
- Cold accommodation (that is less than 15ºC) due to draughts and inadequate heating of weaner facilities – pigs do not gain full thermal control until around 10 weeks-old.
- Poor hygiene, with inadequate cleaning of the weaner facilities – a high infectious dose is generally required for E. coli disease to be a full clinical problem. Therefore many at-risk buildings have been used for pig farming for some years.
- High protein diets and rotavirus infections may make the intestine more favourable for E. coli colonisation.
Also, specific enterocyte receptors for the fimbrial attachment factors are related to pig genetics. Therefore some modern lines of pigs are considered much more susceptible to outbreaks of E. coli disease, such as oedema disease.
Both of these E. coli diseases have had a marked recent resurgence of cases in Europe following political moves to reduce usage of zinc oxide in pig feeds. Zinc oxide has previously proved very effective at controlling disease on pig farms, and outbreaks now often follow its removal from the weaner pig feed ration.
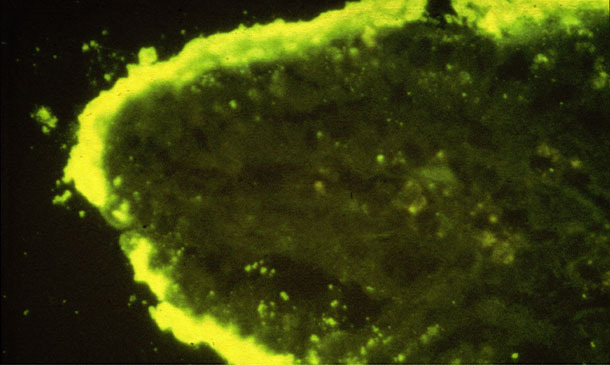
Figure 1. Dense colonies of E. coli attached on an intestinal villus (IFA).
Diagnosis of post-weaning colibacillosis and oedema disease
The examination of fresh pigs at autopsy is required. The autopsy of ETEC diarrhoea cases usually shows dilated, congested thin small intestine loops filled with the watery yellow diarrhoea (see Figure 2), with dehydration and reduction of body fat. Histological lesions are minimal – it is a biochemical lesion, but large numbers of coliform bacteria may often be seen attached to villi, see Figure 1. The collection of appropriate samples for pathology examination requires the samples to be placed into buffered formalin less than 30 minutes after death. Interpretation of any gut samples from animals that have been dead for over one hour is highly problematic.
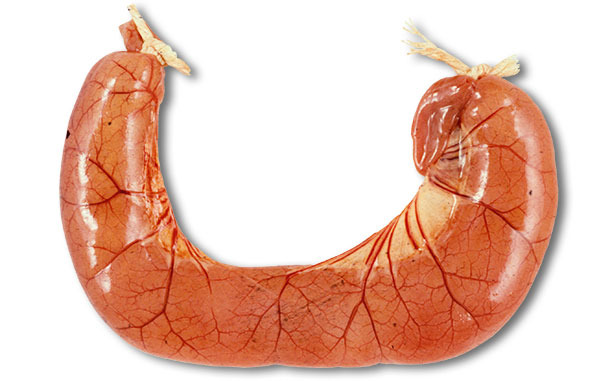
Figure 2. The autopsy of ETEC diarrhoea cases usually shows dilated,
congested thin small intestine loops filled with the watery yellow diarrhoea.
In oedema disease, there is oedematous swelling in the eyelids, larynx, stomach and colon. The distension of the stomach submucosa by clear gelatinous fluid occurs across the greater curvature and in the colon the swelling often is very noticeable in the mesocolon in the spiral flexure. Histologic lesions of arteriopathy with vascular protein leakage occur in these organs and the brain.
Bacterial culture of the intestine can confirm the presence of high populations of E. coli which should be checked for haemolysis, toxins and sensitivity to antibiotics. Unlike Salmonella, the hundreds of various E. coli serotypes are numbered (not named). Fimbrial antigen F4 (K88) is the most common strain in diarrhoea cases and can colonise the entire small intestine. F18 is the most common strain in oedema disease cases. Specific agglutination reagents or PCR tests are available to confirm the attachment proteins involved.

Management and control of oedema disease and colibacillosis outbreaks
It is important to know the history of the disease on the farm and antibiotic sensitivities to the E. coli present. Sick pigs should be treated individually and group treatment applied to the pigs by water medication. If pigs appear dehydrated, then electrolytes should be provided in a separate drinker supply. Antibiotics often used for the treatment of E. coli are apramycin, neomycin, tiamulin and sulphonamides. Re-introduction of in-feed zinc oxide at 2,500 ppm of zinc per tonne may be necessary to prevent further outbreaks. It is necessary for this compound to be placed in feed for at least two to three weeks after weaning. The temperatures, air flow, draughts and fluctuations in the weaner housing must be addressed, as 15ºC is not enough for weaner pigs at 3 weeks-old. Similarly, it is vital to address hygiene of this housing, related to stocking density - group sizes and mixing related to proper pen cleaning. Some farms develop poor hygiene practices related to usage of messy creep feed bowls in weaner pens. Some lines of pigs have been developed that lack the F4 or F18 receptors. However, there can be considerable problems assimilating these lines of pigs into mixed commercial farms, so they have not been widely adopted.
The use of vaccination for post-weaning colibacillosis has re-emerged in the past few years. The time needed for development of vaccine-derived protection of recently weaned piglets requires vaccine delivery in early neonatal stages. For example, protection against oedema disease requires injection at 4-days-old to generate antibodies against shigatoxin Stx2e, prior to exposure after weaning.






