What is gene editing?
Gene editing allows precise changes to be made to the genome of organisms. This involves making modifications by precisely adding, changing, or removing nucleotides from the DNA sequence. Among the available techniques, CRISPR-Cas9 technology has gained great popularity due to its high efficiency, accuracy, and relative simplicity of use.
How does CRISPR-Cas9 work?
- A guide RNA is used, which is a small sequence of 20 nucleotides that matches the part of the DNA to be modified.
- This guide directs the Cas9 enzyme to the exact localization where the two DNA strands should be cleaved (Figure 1).
- After the cleavage, the organism attempts to repair the DNA by a process called non-homologous end joining (NHEJ). During this repair, some base pairs may be added or lost at the cleavage site.
- These changes in the DNA can alter the reading frame of the gene, leading to changes in the amino acid sequence of the protein that the gene produces. As a result, the protein may become defective or nonfunctional. (Figure 2).
|
Remember that proteins are composed of long chains of amino acids, which are the basic units of proteins. There are 20 different types of amino acids, each of them encoded by a specific sequence of three nitrogenous bases in DNA, known as triplets or codons. These bases can be adenine, cytosine, thymine, or guanine.  When the reading frame of the DNA (i.e., the way the bases are grouped into triplets) is altered, the codon sequence also changes. This can result in the incorporation of incorrect amino acids during protein synthesis, or even cause the occurrence of a stop codon, which interrupts protein synthesis (Figure 2). |
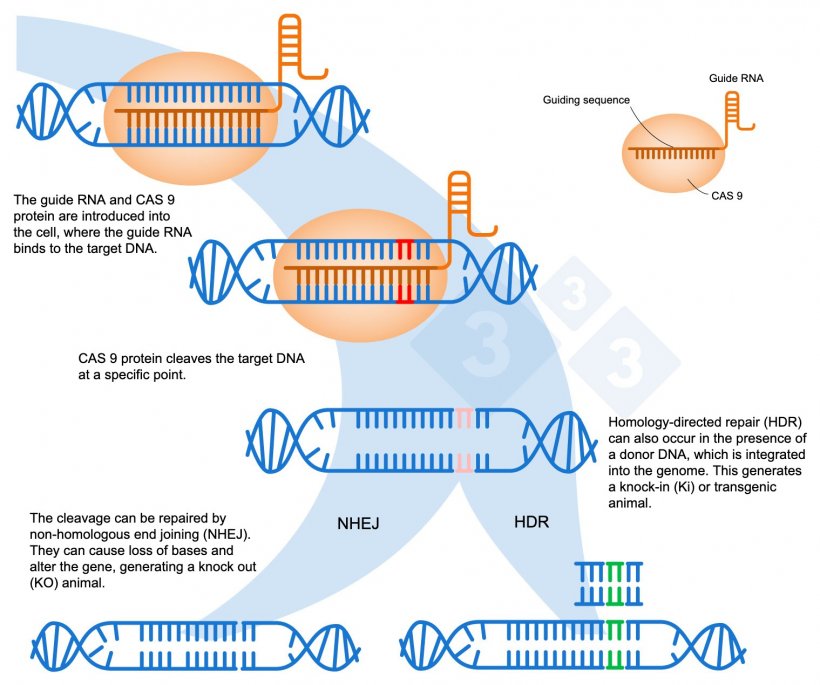
Figure 1. Gene editing using the CRISPR-Cas9 system. A guide RNA (sgRNA) recognizes a specific genomic region, which targets the DNA endonuclease Cas9. This enzyme cleaves the two DNA strands at the precise location. Adapted from: https://es.moleculardevices.com/applications/gene-editing-with-crispr-engineering
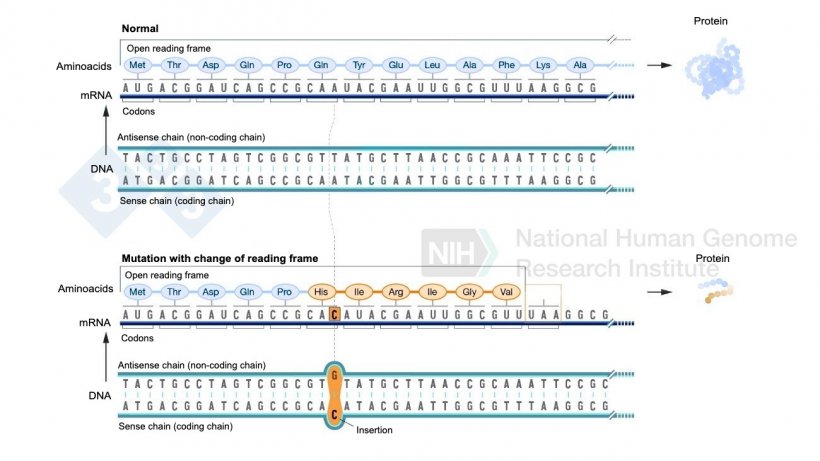
Figure 2. Example of a nucleotide insertion that involves a change in the reading frame and results in different amino acids being formed than normal and finally a stop codon that stops the formation of more amino acids for that protein. Source: https://www.genome.gov/es/genetics-glossary/Mutacion-con-cambio-del-marco-de-lectura
Knock-out animals: when changes in the DNA disable a protein
An animal is considered knock-out (KO) for a specific gene when genetic changes disable the function of a protein. These changes are similar to mutations that could occur naturally through spontaneous processes, but in this case, they are made in a controlled and directed manner. Therefore, it is impossible to distinguish whether a mutation in an animal occurred naturally or was induced in the laboratory.
| It is important to note that genetic material from other species is NOT introduced in this process, so KO animals created by this technique are not considered transgenic. |
An example of a KO animal is a PRRS virus-resistant animal. In order for PRRSV to infect the pig, it needs to bind to a specific receptor present in the alveolar macrophages of the pig's lungs. This receptor is encoded by the SRCR5 domain of the CD163 gene. If this receptor is modified or deleted by gene editing, the virus can no longer bind to the cells, preventing infection and making the pig completely resistant to PRRSV. This genetic resistance can be inherited by offspring, ensuring that future generations will be resistant to PRRS.
Knock-in animals: Introducing new DNA strands and new genes
An alternative in gene editing is to take advantage of when DNA is cleaved by Cas9 to introduce a new DNA strand. In doing so, the cell can incorporate this new genetic material through a process called homology-directed repair (HDR. Figure 1). This allows a defective DNA sequence to be replaced with a functional one, correcting the gene to make it functional again or altering the reading frame to create a KO animal, in which a gene has been inactivated. In addition, this technique makes it possible to insert a new additional gene, which it did not have before, into the organism's genome, known as knock-in (KI) or transgenic.
| When the gene introduced comes from another species, a genetic combination is created that does not occur naturally. This type of organism is called a transgenic animal since it has genes from another species integrated into its DNA. |
An example of transgenic pigs would be those to which the UPC1 gene has been inserted, which is not naturally present in this species. This gene allows them to better regulate their body temperature, which makes them better adapted to withstand low temperatures. As a result, pigs accumulate less subcutaneous fat to maintain their body temperature, which increases the percentage of lean tissue (Figure 3).
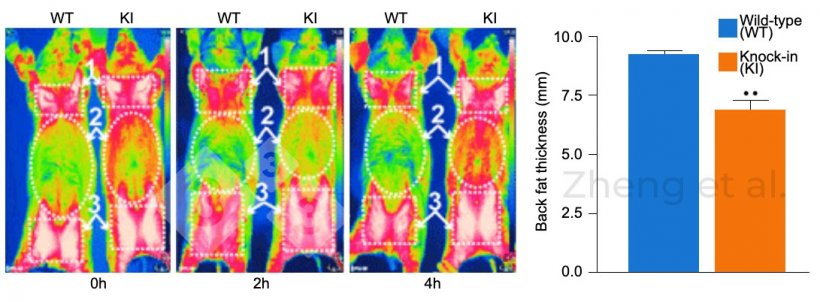
Figure 3. Infrared images were taken at 0, 2, and 4 hours of cold exposure in 6-month-old pigs. Backfat thickness in 20-kg piglets. Source: Zheng et al. (2017). “Reconstitution of UCP1 using CRISPR/Cas9 in the white adipose tissue of pigs decreases fat deposition and improves thermogenic capacity.” Proc Natl Acad Sci U S A 114(45): E9474-E9482.
Base editors: a precise tool in gene editing
A more recent alternative in gene editing is the use of base editors, which are specialized enzymes that, instead of cleaving DNA, precisely exchange a base pair at a specific location in the genome. This process makes it possible to make targeted changes to the DNA without creating breaks.
This type of substitution can have several effects. In some cases, the change introduces a stop codon, which stops the production of the protein prematurely, leaving it incomplete and possibly non-functional. In other cases, the change alters an amino acid in the protein (known as a missense mutation), which can modify its structure and affect its function. In both cases, these alterations can compromise the protein's functionality.
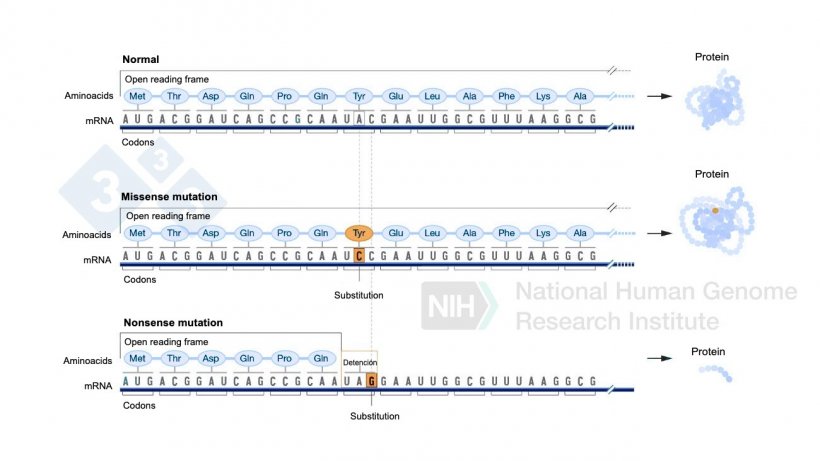
Figure 4. Use of base editors to generate point mutations in the genome.
Are all gene-edited pigs the same? Normative Regulation vs. Technical Development
It is important to have a technical understanding of the different types of gene editing, since the regulations that apply, or should apply, vary in each case. Currently, most of the world's legislation strictly limits the use of transgenic animals and the commercialization of their products. However, progress is being made in distinguishing between genetically edited animals and transgenic animals, which could lead to differentiated regulations. Technically, these two types of genetic modifications are totally different.
Regulations in the European Union concerning genetically edited plant products, known as new genomic techniques (NGT), are already under review. This could allow these plants to be exempted from the laws that regulate transgenics called genetically modified organisms (GMOs). However, to date, gene-edited animals are still subject to GMO legislation, which limits their development and future commercialization.

Project funded by Fundación Seneca 22065/PI/22.


