Bacterial resistance against antibiotics is a global challenge. A massive use of antibiotics in industrial pig production is a major cause of the current rise in resistant bacteria. Particularly gut infections demand large amounts of antibiotics, thus improving the state of pig gut health will have major impact on antibiotic consumption.

Microbial life in the intestines is a key player in gut health status
Gut health depends on many factors like digestion and absorption of nutrients, immune status, gastrointestinal illness and composition of the gut microbiota. The gut microbiota is a complex and dynamic inner community of more than 1000 different species, and is essential to gut health of all mammals. This microbiota has the potential to modulate many of the factors that affect health. Most of the bacteria are essential companions that provide great benefit to the host, including degradation of nutrients, activation of host genes involved in nutrient absorption, vitamin supplementation, immune development and prevention of pathogen colonization. This means that balancing the composition of these inhabitants is an important tool to help control gut health.
Colonization of the gut depends on a wide range of genetic and environmental factors, which we only just recently have started to understand. We need to gain further understanding of how colonization is controlled, and which molecular mechanisms are used, to potentially modulate the microbiota to benefit gut health.
Genetics are important to control which microorganisms inhabits the gut
Genetics have the potential to regulate bacterial colonization, and in particular a natural variation in the α1,2-fucosyltransferase 1 (FUT1) gene seems to play an important role. The FUT1 variation controls infections with F18 fimbriated enterotoxigenic E. coli (ETEC F18) (Meijerink et al. 2000). ETEC F18 is responsible for a considerable part of gut infections, particularly in newly weaned pigs. Further information about how this genetic variation improves gut health is of great value, and could potentially help to reduce the need for antibiotic treatment in pig production.
FUT1 controls the assembly of structures important to bacterial colonization
FUT1 is an enzyme that assists in the construction specific sugar molecules by transferring fucosyl units to a carbohydrate assembly line. All cells are coated with specific recognition molecules, to allow cells to interact specifically with each other. Most commonly, these consist of specific sugars that are linked to specific proteins or lipids. Such sugars are also very important for microbial life in the intestines, serving both as microbial anchor sites to the gut wall, and as an important nutritional source for the microorganisms (Fig. 1). A change in the availability of these sugars may very well have fatal consequences for some bacteria.
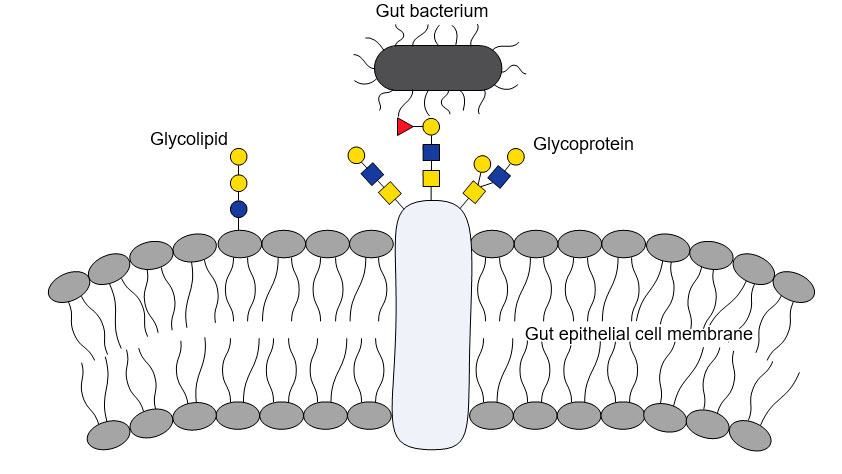
Figure 1: Bacterial-glycan interactions are important to bacterial colonization of the gut, as bacterial molecules adhere to specific glycans on host cells.
Our recent study showed that a specific variant of the FUT1 gene controls expression of some fucose-sugars in the intestine (Hesselager et al. 2016). These specific sugar structures are also known as histo-blood group antigens, and have previously been observed to impact bacterial adhesion (Gagneux and Varki 1999), but it has not previously been demonstrated that these specific sugars are coupled to gut cell proteins. Thus, our finding strongly supports that fucose-sugar-proteins provide important opportunities for manipulating bacterial colonization of the gut.

Can genetic selection improve pig gut health?
We have now established a direct chain of events connecting a naturally occurring genetic variation to specific biochemical changes in intestinal sugar molecules, and to a direct impact on health and pathogen susceptibility (Fig. 2). This knowledge opens the door to further understand genetic influence on gut health, and for exploiting this knowledge to improve pig health by genetic selection.
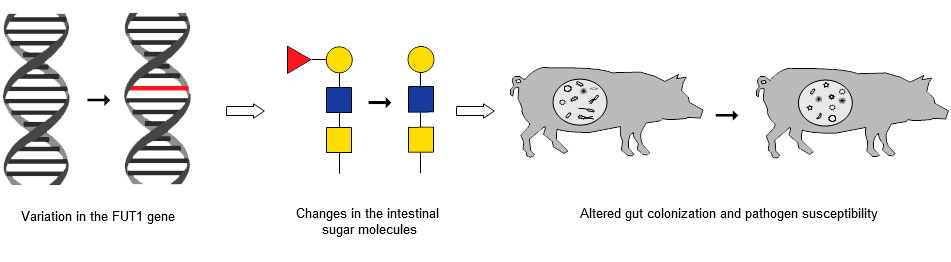
Figure 2: The chain of events that links a known variation in the FUT1 gene to changes in intestinal sugar molecules and in altered gut colonization and pathogen susceptibility.
The FUT1 genetic variation provides resistance to ETEC F18, however, as intestinal sugars are commonly used as microbial attachment sites or nutritional source, other microorganisms also playing important roles for animal health may be affected as well. This means that, to fully understand the health impact of this genetic variant, we need a more thorough understanding of how colonization of other bacteria are affected, and in particular the beneficial members of a healthy gut microbiota.
We conclude that genetic selection provides a powerful tool to improve and maintain gut health in industrial pig production, and may in the future become a more sustainable alternative to the currently massive need for antibiotics.




