The disease caused by the PRRS virus (PRRSV) is one of the globally most important diseases in swine production. During the last 40 years, discussions around PRRSV have been very intense, and the clinical implication of this disease continues to grow. The cost of PRRSV in Europe has been reported to vary between €75.72 and €650.09 per sow basis, including all production phase costs. In the United States, a recent update was provided at the 2024 IPVS Congress, whereas the annual cost of PRRSV has increased from $664 million to $1.2 billion for the US swine industry. Nevertheless, PRRSV keeps genetically evolving generating more aggressive strains like the L1C.5 in the United States or the Rosalia in Europe. In the US, a 21-year trend in sample types for PRRSV monitoring reveals that population-based samples such as oral fluids and processing fluids (PF), utilized for classifying breeding herds by PRRSV status, have become the most commonly used samples for PRRSV RT-PCR testing over the past years (Figure 1). This shift allows for the sampling of more animals and enhances the detection of PRRSV, even at low prevalence.


Producers continue to battle PRRSV, with a recent study showing that the practices implemented across breeding herds to control or eliminate PRRSV are inconsistent.
Moving away from weekly batch management
The implementation of production flows managed in batches usually moves the production of breeding herds from a weekly basis to intervals determined by the batch model adopted. Usually, batches move production flow from a weekly basis to regular intervals of two, three, four, five, or seven weeks. Some advantage of such production flow organization relies on concentrating the labor activities in one week, facilitating the implementation of all-in-all-out for all farrowing rooms, and a better organization of production flow, e.g., quick filling of a nursery site and logistics organization. The question that still remains is:
does moving away from weekly batch management to farrowings every 3,5 or 7 weeks provide a competitive advantage when we talk about the control and elimination of PRRS?
A comparison of a study conducted on breeding herds operating on a weekly basis with another conducted on farms operating on a four-week batch system (n = 27) reveals some light on the advantages of batching farms. In both studies, the breeding herds adopted a load-close-exposure (L-C-E) program in response to PRRS outbreaks.
The L-C-E relies on:
- Loading the herd with incoming gilts.
- Closing the herd to new introductions of replacement animals.
- Exposing all the animals to a PRRSV strain by adopting an specific program.
In both studies, a commercially available modified live vaccine was used for the exposure of the whole herds.
- The first study used serum to monitor breeding herds undergoing elimination following the AASV proposed guidelines that relied on four consecutive samples every 30 days from 30 due to wean piglets with a negative RT-PCR testing result for PRRSV.
- The second study followed a modified approach and used Processing Fluids to detect PRRSV circulation in herds undergoing virus elimination. After two consecutive negative results (i.e., two batches or eight weeks) were obtained in PF, serum was collected from 60 due-to-wean piglets and tested in pools of 5:1 by RT-qPCR for PRRSV RNA. Herds were considered stable when a negative result was obtained on the due-to-wean sera, along with two previous consecutive negative results on PF samples. After a negative result on due-to-wean sera, the farm was re-opened, and sentinel PRRSV naïve gilts were introduced. After 30 days post entry, sentinel gilts were tested by serum to investigate the presence of PRRSV antibody, and if the results were negative, the herd was declared negative.
When comparing both studies, it could be identified that:
Time to stability (TTS): the median time for the farms operating in the four-week batch system was 27 weeks vs 32 weeks for the farms operating in weekly batches ⇒ five weeks shorter TTS for batched farms. A more recent study reported a median TTS of 38 weeks, which would make such a comparison even more dramatic.
Gilt Reintroduction: Farms operating in the four-week batch system were able to reintroduce gilts with a mean time of 33 weeks, whereas farms operating on a weekly farrowing basis introduced gilts at a mean time of 45 weeks. The difference between both operation strategies was a 12-week advantage for gilt introduction in the batch farms (Figure 2).
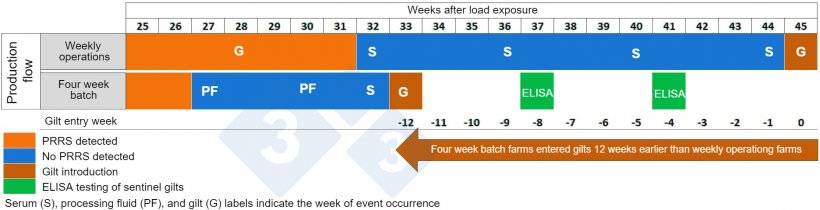
Preliminary analyses of the PRRS Outbreak Management Program (POMP) using more recent data have shown equivalent results. POMP is an epidemiological database to track PRRS outbreaks in breeding herds. The goal of the program is to evaluate different control and elimination practices during a PRRS outbreak and how those practices impact three different outbreak recovery metrics. The metrics are:
- Time to Stability (TTS): number of weeks it takes for the farm to produce 13 consecutive weeks of PRRSV PCR-negative pigs (At weaning).
- Time to baseline production (TTBP): number of weeks for the farm to wean the same volume of piglets as prior to the outbreak.
- Total Piglet losses per 1,000 sows (TL): number of lost weaned pigs between the outbreak and TTBP adjusted to 1,000 sows, so that comparisons can be made between breeding herds of different sizes.
Thus, POMP preliminary analysis has demonstrated a statistically significant improvement in TTS of 25 weeks faster for farms that use batch farrowing compared to weekly farrowing (Figure 3). Additionally, the batch farms also were associated with a 32% reduction in total losses when compared to continuous farrowing farms.
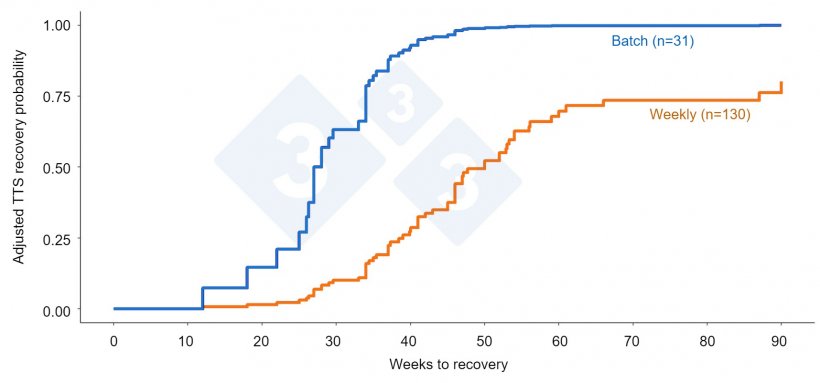

Batch farrowing likely helps break the pathogen transmission cycle by avoiding infection of younger populations (younger batches) from the older pig populations (older, closer to weaning age, batches). Logic and common sense allow us to speculate that batch farrowing will also reduce the transmission of other infectious agents, including porcine enteric coronaviruses, influenza A virus, E. coli, just to list a few. In conclusion, results from these epidemiological studies support the hypothesis that implementing batch farrowing, which is essentially ‘all in-all out’ in the farrowing house, makes it hard for pathogens to sustain long-term transmission in the herd.








