The gas transfer velocities from the liquid phase to the atmosphere is proportional to the difference between the concentration in the liquid phase and that which it would have if it were in equilibrium with the gas phase. The equilibrium concentration is that at which there is no transfer, i.e., no emissions. A gas that is not very soluble (low equilibrium concentration, as in the case of CH4) will tend to escape rapidly into the gas phase, whereas otherwise (as in the case of NH3) it can maintain high concentrations in the liquid phase without emissions.
Not all ammoniacal nitrogen is in the form of NH3, rather it is mostly in the form of non-volatilizable NH4+. As pH or temperature decreases, more nitrogen will be in the form of NH4+ and more ammoniacal nitrogen (sum of NH3 and NH4+) will remain in the liquid medium. If the concentration of NH3 in the gas phase above the slurry increases beyond the usual values in the atmosphere, so will the equilibrium concentration in the liquid. Thus, at pH 7, a temperature of 20°C, and a concentration of 50 ppm NH3 in the gas phase above the slurry, the equilibrium concentration of ammoniacal nitrogen in the liquid is 5,260 mg N/L, while at pH 8 this concentration is 540 mg N/L. To prevent emissions, and to maintain higher concentrations in liquid media, a pH of 7 is better than pH 8, and if the pH is lower than 7 it is better still. The lower the NH3 concentration in the air layer above the slurry surface (average concentration in the earth's atmosphere of 1 - 5 ppb), the lower the equilibrium concentration and the faster the emission will be. This would be the case in the event of wind or any other gas dispersion phenomena. Covering the lagoon, even slightly, to protect it from dispersion or to decrease the surface area of liquid/gas exchange, will have an effect in reducing NH3 emissions. Not so with CH4, which will escape to the atmosphere under any circumstances, unless the lagoon cover is completely airtight.

CH4 emissions depend on the time during which anaerobic microorganisms decompose organic matter, whose activity increases with temperature. The figure shows the CH4 emission factors according to the 2019 IPCC (Intergovernmental Panel on Climate Change) manual for temperate climates and warm climates, either humid or arid. Temperate climates correspond to average annual temperatures between 15°C and 25°C, and warm climates to higher temperatures. The IPCC manual indicates that in order to estimate emissions in the pits under the slat, the environmental conditions in the animal enclosure- a warm, humid environment- should be considered, not the outside conditions. The latter has been experimentally proven. Simply removing the slurry from the barns as soon as possible to an outdoor lagoon can reduce emissions, e.g. from 57% to 24% in case of storing for 3 months (see figure).
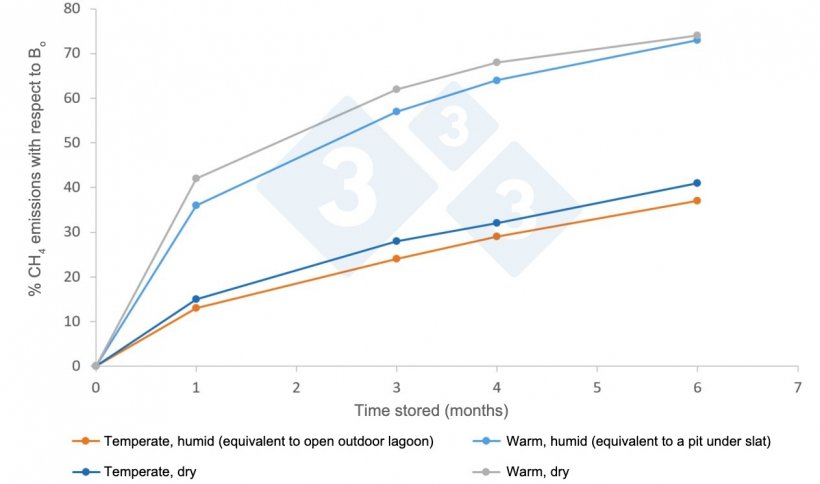
Figure. Average factors of CH4 emission (%) to the atmosphere according to climate and storage time, according to the IPCC 2019 manual. Bo is the maximum potential to emit, which for pig slurry is 0.42 m3 CH4/kg SV under normal conditions of pressure and temperature (0 degrees C and 1 atm).
To prevent NH3 emissions, different techniques can be applied in order of priority:
- Improve diets and protein and amino acid digestibility. The higher the feed efficiency, the lower the concentration of nitrogen in the slurry and the slower the transfer velocities.
- Remove slurry from barn pits as often as possible, every day rather than every week, and every 6 hours rather than every 24 hours. Although the IPCC manual estimates average nitrogen emission values of 25% (range of 15% - 30%) of excreted nitrogen for pits under slats, and 48% (15% - 60%) for external lagoons without a natural crust, the lagoon allows for covering and preventing emissions (lowered to 3% - 12% according to the IPCC manual), which is not achievable in pits. It also prevents the animals from inhaling ammonia, which has health and production benefits.
- Cover outdoor lagoons with a material that reduces the contact area between the surface of the stored slurry and the atmosphere. For example, solid floating pieces that fit together to completely tessellate the surface of the slurry. Covering the lagoon with an impermeable membrane will also prevent the emission of CH4, a gas that will accumulate under the membrane and must be burned periodically in a flare or boiler, or for energy use, to prevent the membrane from rupturing due to excess pressure. This excess pressure will never be due to NH3, since once the equilibrium concentrations between liquid and gas phases are reached, its emission will stop.
- Reduce the pH of the slurry to below 7 by adding acid and using equipment specially designed for this purpose. There is also equipment to acidify in the slurry transport and agricultural application tank to prevent the loss of NH3 during applications.
To prevent CH4 emissions, the following techniques can be applied:
- Improve the digestibility of diets and prevent feed loss to slurry. Although there is still no evidence-based information, a higher efficiency in animal feeding should imply a lower energy potential of volatile solids (VS) in the slurry and, therefore, a lower emission potential value Bo.
- Remove slurry from the barn pits as soon as possible. As shown in the figure, this practice has a considerable effect on reducing CH4 emissions.
- Adopt a solid/liquid separation as soon as possible after the slurry is removed from the barns. The stacked solid fraction has emissions around 4% - 5% Bo according to IPCC, given the volatile solids it contains, and the liquid fraction will emit according to the corresponding value in the Figure, but for a lower concentration of VS and, therefore, lower emissions.
- Cover the outer lagoon with an impermeable membrane to prevent emission into the atmosphere. Such a lagoon must be designed so that it never empties completely, so that no air enters, and it must include safety mechanisms to avoid excess pressure as well as a flare to periodically burn off the accumulated gas. A better alternative is to use gas as boiler fuel.
- Plan a biogas plant, whether individual or collective, utilizing the gas as an energy source. The slurry should enter the plant as soon as possible to take advantage of its full energy potential. The digestate lagoon must be covered to prevent NH3 emissions, since the concentration of ammoniacal nitrogen is higher than that of the original slurry, due to the decomposition of proteins.










