Stress-associated immune response changes in the gastrointestinal tract
Balance must be maintained between the animal's organism and the surrounding ecosystem. Prevention of chronic inflammatory processes on the enormous surface of the intestinal mucosa (about 300 m2 in a 100 kg fattener) is particularly important.
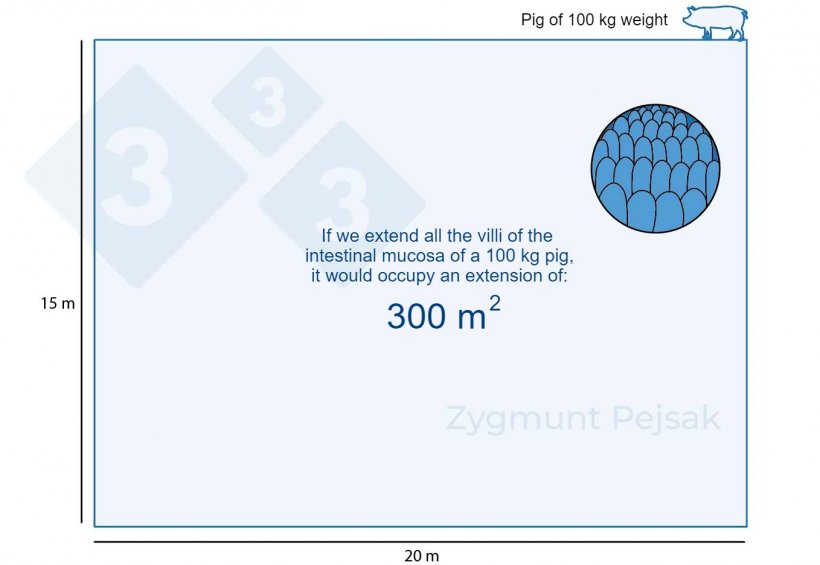
Figure 1. The intestinal mucosa of a 100 kg fattening pig occupies about 300m2

Gut-associated lymphoid tissue (GALT) found just beneath the epithelium of the gastrointestinal tract is the largest bastion of the immune system. The function of the immune cells that are part of GALT is to react quickly and strongly to invasion by microorganisms or to their toxins and to limit the scope of reaction against non-pathogenic factors to prevent excessive immune system activity and, consequently, excessive damage to the organism by the inflammatory reaction (Burkey et al., 2009).
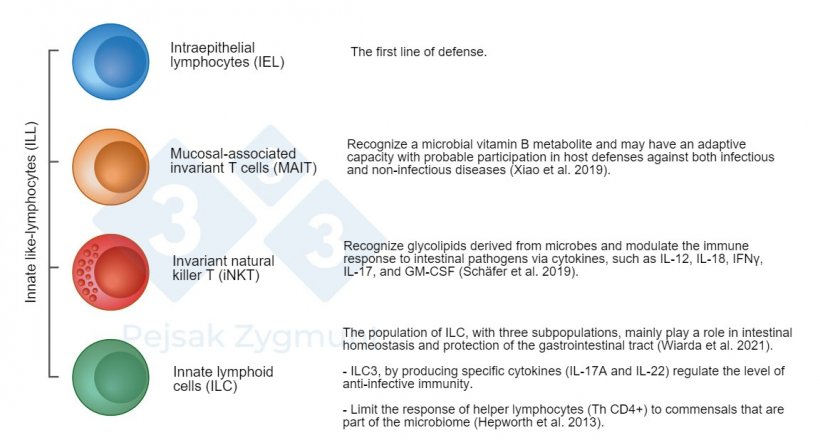
Figure 2. Among GALT we can distinguish a few pivotal populations: Innate like-lymphocytes (ILL) are located in the small intestine and, to a lesser degree, in the large intestine, play an important role as producers of cytokines, cytotoxic molecules, and antimicrobial peptides (Hepworth et al., 2013; Schäfer et al., 2019; Wiarda et al., 2020; Wiarda et al., 2021; Xiao et al., 2019).
An immune response is triggered when immune system cells recognize a pathogen which causes a rapid release of pro-inflammatory cytokines, TGF beta, and other mediators into the surrounding tissues and bloodstream. Cytokines give the effector cells information about the site of infection and the latter help remove the pathogen.
Stress related to the animal’s environment impairs the intestinal barrier and induces immunosuppression of intestinal immune responses due to the up-regulation of IL-10 (Li et al., 2017), which may decrease production efficiency and increase the incidence of diseases (Peng et al., 2021).
Immune stress and its influence on the body and production
Immunological stress is defined as the long-term immunological mobilization of the animal's organism, related to defense against constant pressure from unfavorable environmental conditions, including pathogens. There is still an incomplete understanding of the mechanisms related to long-term immune stress, which determine the negative effects on the animal's development, including higher morbidity and mortality, increased feed conversion rate (FCR), and weight differentiation between groups of same-age pigs. It is known, however, that the innate immune system is part of the first line of anti-infective defense of the host. The barriers of the epidermis and mucosa epithelium constitute the first barrier to the pathogenic factor.
When the pathogen is strongly pathogenic or present in large numbers, or when the barriers are damaged, it is possible to break these barriers and infect the host. Microorganisms that have broken such barriers are recognized by immune system cells through pathogen-associated molecular patterns (PAMPs) derived from bacteria, viruses, fungi, and protozoa by Toll-like receptors (TLRs). TLRs, which are expressed on macrophages, dendritic cells, mast cells, and NK cells, as well as on T and B lymphocytes, distinguish pathogens from dead and inactive (debris) elements (Mair et al., 2014). The activation of TLRs is a pivotal signal for the activation of both innate and acquired immune responses and for combating the pathogen in accompaniment with a massive proliferation of immune cells and production of immunoglobins, cytokines, chemokines, acute phase proteins, and many others.
During immune stress, the metabolic priority is mainly the production of APP by the liver. Acute phase proteins include proteins whose concentration in plasma increases by at least 20% after tissue damage. The priority action of APP is to restore the body's homeostasis. A negative consequence of APP activity is increased catabolism leading to weight loss. This is related to the persistently high level of cytokines secreted by stimulated leukocytes. Weight loss is exacerbated by the overproduction of cytokines which contribute to changes in mood and behavior leading to weakness and sometimes even a lack of appetite (Dantzer et al., 2008; Chaytor et al., 2011; McKim et al., 2018; Munshi et al., 2019).
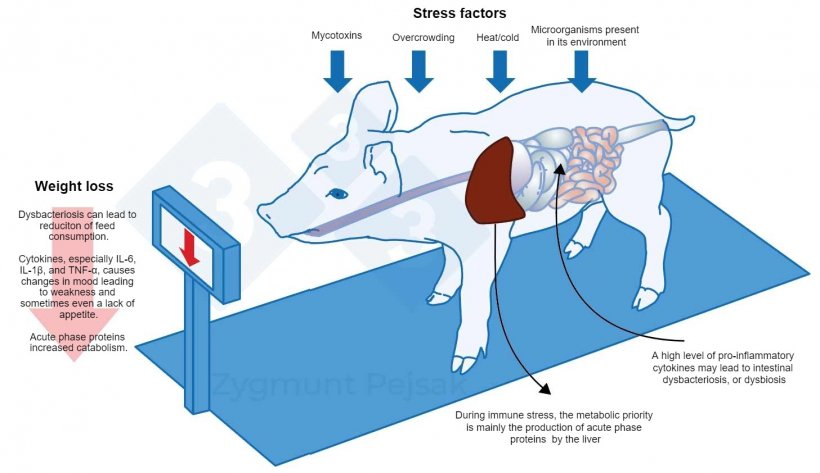
Figure 3. An additional cost of a long-term alert is the deterioration of appetite and, consequently, a prolonged fattening period. Since the pressure from environmental stressors affects individual animals differently, there will be a differentiation in the weight of weaners or fattening pigs of the same age. Weight differentiation among animals of the same age may have serious economic consequences. Also, especially in young animals, a diet that does not contain nutrients essential for strengthening the anti-infectious resistance may make the animals more vulnerable to infection.
Any disturbance of homeostasis due to the impact of long-lasting stress on the organism weakens its immune defense and increases its sensitivity to pathogenic microorganisms. Improper feeding of animals may also worsen the effects of imbalance. Especially in young animals, a diet that does not contain nutrients essential for strengthening the anti-infectious resistance may make the animals more vulnerable to infection by pathogenic microorganisms. An appropriate diet can support the pig's immune system, and in sows can even have a positive effect on the immune systems of its piglets (Werner et al., 2014; Rudar et al., 2016). On the other hand, mycotoxins naturally present in corn may result in an altered immune response with systemic inflammation and partial liver damage, causing a reduction in the growth of pigs (Chaytor et al., 2011).
An excessive, long-lasting immune alert leads to inflammatory changes in the intestines, and a high level of pro-inflammatory cytokines may lead to intestinal dysbacteriosis, or dysbiosis (a microbiological imbalance in the intestines). This may cause undesirable consequences affecting the entire body (Dobson et al., 2000). Changes in the composition of the microbiome can be qualitative, such as a decrease in the diversity of the intestinal flora, and/or quantitative, such as changes in the number of individual bacteria.
Another consequence of a long-term immune alert may be the periodical deterioration of appetite and, consequently, a prolonged fattening period. A deterioration in FCR is often observed. Since the pressure from environmental stressors affects individual animals differently, there will be a differentiation in the weight of weaners or fattening pigs of the same age, which can have serious economic consequences.
In conclusion, minimizing the exposure of pigs to stress, limiting the survival and multiplication of pathogenic and conditionally pathogenic microorganisms, as well as improved management, can significantly enhance the functioning of the pig’s immune system and, consequently, reduce the costs of production by improving feed conversion and reducing treatment costs.





