Stress is most commonly defined as a biological phenomenon consisting in an organism's reaction to excessive physical or mental demands. Stress activates the general adaptation syndrome (GAS), characterized by specific hormonal activity and pathological changes. The main sign of a stress reaction is an increase in the output of glycosteroids from the adrenal cortex.
In intensive pig production, stress may be caused by disturbances, such as those related to the excessive density of animals, frequent movement, or long transport, which disturb the balance between the environment and the animals (Nawroth et al., 2019). Due to connections between various physiological mechanisms, stress may negatively affect not only immunity and consequently health, but most of all reproduction (Dobson et al., 2000).

There are two reactions to stress, depending on the behavior of the affected individual: an active reaction, which is an attack or flight, and a passive reaction, consisting in immobility and depression during longer-lasting stress (Henry and Stephens, 2013). The adrenaline and noradrenaline rush is characteristic of an active reaction. During a passive reaction, increased levels of corticosteroids are observed (Menash, 2013).
Influence of stress on the immune response
The mechanisms related to stress that determine its impact on the immune system are still not fully understood. However, the mutual influence of the nervous and immune systems is largely known. The major pathways involved in these interactions are the sympathetic nervous system (SNS) axis and the hypothalamic–pituitary–adrenal (HPA) axis. These systems communicate with each other through two axes. The SNS axis involves the sympathetic nervous system and the adrenal medulla. The sympathetic nervous system is part of the autonomic nervous system, which activates internal organs and is responsible for the fight-or-flight response (Tindle and Tadi, 2022).
Figure 1 illustrates the response in a pig when the sympathetic system is triggered by an acute stressor. The activation of the sympathetic system in pigs can be measured by Chromogranin A (CgA), which is an acidic soluble protein belonging to the granins family (Escribano et al., 2013).

Figure 1. After a stressor is triggered, the sympathetic system stimulates the adrenal glands to release adrenaline and noradrenaline. The activation of the sympathetic system in pigs can be measured by Chromogranin A (CgA).
The other axis, HPA, is activated minutes or hours after stress occurs (Lightman, 2008), illustrated in Figure 2. The cortisol produced by this stress response has been widely used as a marker of stress in swine, also as a noninvasive test from saliva (Hillman et al., 2008; Ruis et al., 1997; Valros et al., 2013).
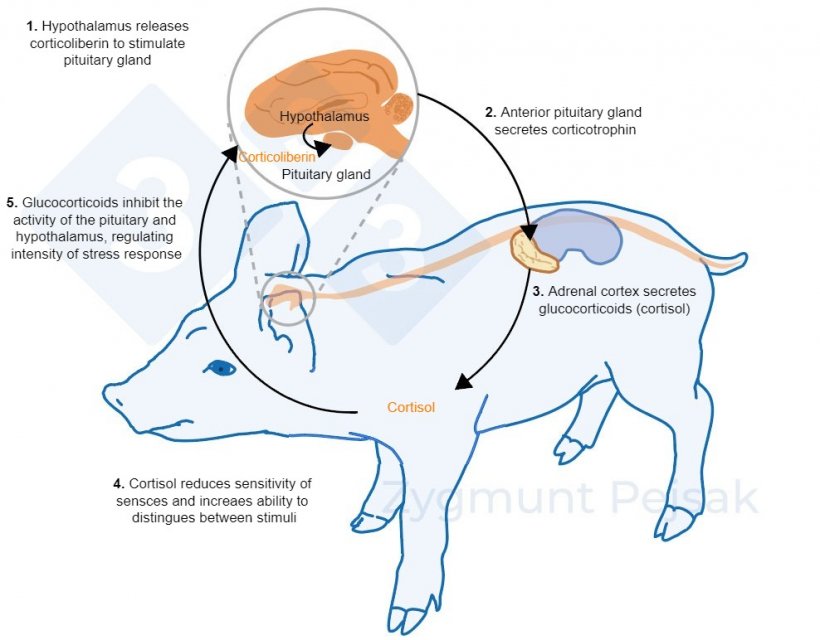
Figure 2. Minutes or hours after stress occurs, the hypothalamic–pituitary–adrenal (HPA) axis is activated. Pituitary gland secretes corticotrophin, which, reaching the adrenal cortex, causes the secretion of glucocorticoids, including cortisol. Glucocorticoids inhibit the activity of the pituitary and hypothalamus, thanks to which they regulate the intensity of the stress response. Cortisol has been widely used as a marker of stress in swine, also as a noninvasive test from saliva.
Glicocorticoids have a negative effect on the immune system. They may have an impact on the innate immune response reducing the number of circulating monocytes and hence also proinflammatory cytokines. Glucocorticoids inhibit leukocyte traffic and thereby the access of leukocytes to the site of inflammation (Strehl et al., 2019). A strong and prolonged stress inhibits the humoral and cellular response of the immune system which results in "hyperadaptosis," often leading to the manifestation of a disease (Wrona - Polanska, 2008). Details of the effects are outlined in Figure 3. Meanwhile, a short psychological stress, such as the social isolation of piglets, induces a state of cortisol resistance in blood immune cells, which may be an adaptation aimed at maintaining cellular immune responses in the short term (Escribano et al., 2013).
Table 1. There are two reactions to stress: an active reaction, which is an attack or flight, and a passive reaction, consisting in immobility and depression during longer-lasting stress.
| Short stress: active reaction | Prolonged stress: passive reaction |
|---|---|
| A short psychological stress induces a state of cortisol resistance in blood immune cells. | Glucocorticoids have a negative effect on the immune system: |
|
↑ CD8+ lymphocytes ↓ CD4+/CD8+ ratio |
↓ Circulating monocytes ↓ Proinflammatory cytokines IL-2, IL-6, TNF-α, and IL-12 ↓ Th1 lymphocyte differentiation ↓ T cells ↓ IL-2 (necessary for T cells) ↓ leukocyte traffic ↓ humoral response ↓ cellular response ↓ reactivity of lymphocytes ↓ production of lymphocytes (sometimes even activate the process of their apoptosis) |
There are a few immune biomarkers that change immediately after stress, and therefore could be easily used as biomarkers of acute stress (Puppe et al., 1997). Including:
- an increased neutrophil/lymphocyte ratio
- salivary serum amyloid A
- immunoglobulin (Ig A)
- IL-18
Acute stress is commonly associated with a proinflammatory response, which may be helpful in setting the immune system to fight and heal (Dhabhar et al., 1995). In general, chronic or repeated stress reduces immune responses, whereas a single exposure to stress enhances immunity (Dhabhar, 2009). This is also supported by studies on innate immunity in pigs. However, the ultimate effect of a stressor also depends on stress factors. Table 2 outlines a list of acute and chronic stress factors and their responses in pigs. The table also shows that the effect of stress on the immune response may also influence individuals in utero.
Table 2. Stress response depends on the stress factor.
| Stress factor | Response | Study | |
|---|---|---|---|
 |
Short transport (20 minutes) |
↑ some acute phase proteins (APP), haptoglobin, and CRP ↑ neutrophil/lymphocytes ratio |
Dhabhar, 2009; Puppe et al., 1997 |
 |
Long transport (more than 6 h) |
↑ pro-inflammatory cytokines (IL-2, IL-6, IL-12, IL-1β, and IFN-γ) ↓ anti-inflammatory cytokine IL-4 |
De et al., 2021 |
 |
Acute immobilization stress |
SNS stimulation ↑ IL-18 in saliva |
Piñeiro et al., 2007 |
| Isolation of young piglets | ↑ IL-6 mRNA in the hypothalamus | Tuchscherer et al., 2004 | |
| Repeated social isolation in piglets | ↑ cytokines (IL-1β) |
Kanitz et al., 2004; Tuchscherer et al., 2004 |
|
 |
Social stress | Suppress the immune response to a viral vaccine and consequently impairs protection against a challenge infection more in dominant than in subordinate individuals subjected to social stress | Groot et al., 2001 |
| Chronic social stress |
Immunosuppressive influence Significant down-regulation of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-8) probably due to a robust upregulation of IL-10 expression in the ileum and colon |
Bennett et al., 2015 | |
 |
Prolonged stress associated with food intake interruptions | ↑ APP, especially in males | Muneta et al., 2011 |
 |
Fetal heat stress (in utero) | ↑ cytokine response (TNF-α, IL-1β, and IL-6) to lipopolysaccharide (LPS) in fetuses in utero compared with fetuses that are not exposed to heat stress, probably due to an altered metabolic and cortisol response | Johnson et al. 2020 |
| Chronic heat stress | No effect on lymphocyte proliferation |
Bonnette et al., 1990; Morrow-Tesch et al., 1994 |
|
| 14-day exposure to heat and crowding stress |
↑ proliferation of T cells and NK cell cytotoxity No effect on the total IgG concentration |
Sutherland et al., 2006 | |
 |
4-day cold stress |
↓ NK activity ↓ T cell proliferation ↓ Total plasma IgG concentration |
Salak-Johnson et al., 2018 |
 |
Maternal stress |
↓ Serum IgG level in suckling piglets and immunosuppression of B and T cell proliferation No change in NK cell cytotoxity ↓ thymus weight |
Tuchscherer et al., 2002 |
It seems that the ultimate effect of stress on the immune system depends not only on the duration of stress and stressor factors, but also on the individual exposed to this stress. Social stress appears to suppress the immune response to a viral vaccine and consequently impairs protection against a challenge infection more in dominant than in subordinate individuals subjected to social stress (Groot et al., 2001). Generally, socially dominant and submissive pigs showed alterations in the immune function (elevated numbers of neutrophils and decreased antibody production) compared with average pigs. Heat and social stress interact in their effect on the pig's immune system and cause immunosuppression in average animals, although there are also immunological costs to dominant pigs as well (Mc Kim et al., 2018).
Stress is also connected with stereotypies and even destructive disorders, such as tail biting, which may be counteracted by environmental enrichment (D’Eath et al., 2004). What is interesting, environmental enrichment may also affect certain components of the immune system (Luo et al., 2017; Manciocco et al., 2011; Reimert et al. 2014; Scollo et al., 2013; Scott et al., 2006). Some data indicate lower N:L ratios and haptoglobin levels in enriched-housed pigs, which suggests that they are less stressed and their immune system is less activated (Beattie et al., 2000). These results confirm the relationship of stress with the immune system, as well as the beneficial effect of enrichment on pig behavior and welfare. It should be emphasized that a short-term activation of the immune system and pro-inflammatory response may be beneficial, although it has a negative effect in the long term.







