The pH of the medium affects the dynamics of ammoniacal nitrogen, shifting the chemical equilibrium to the soluble ionized form (NH4+) when the pH is acidic, and to the non-ionized, volatile form (NH3) when the pH is basic.
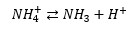

This equilibrium depends on both pH and temperature. Figure 1 shows the percent NH3 of the total ammoniacal nitrogen as a function of these two variables in a solution of ammoniacal nitrogen in water. Although the salinity of the slurry can modify the indicated values, they are a good approximation to illustrate what is presented here.
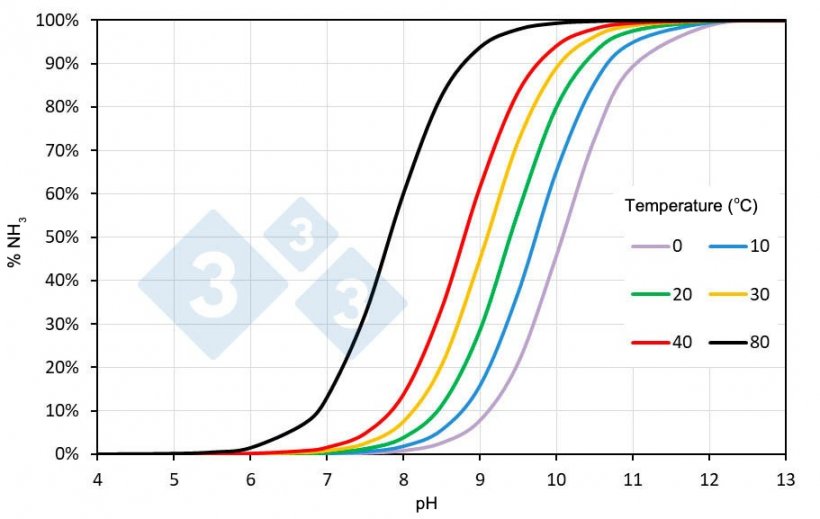
Figure 1. Percent ammonia (NH3) in a solution of ammoniacal nitrogen in water as a function of pH and temperature. The salinity of the slurry can modify the indicated values.
NH3 is a gas, which volatilizes into the atmosphere, while the ionized form (NH4+) remains soluble. Acidifying the slurry, by adding an acid, has the advantage of shifting the equilibrium towards the soluble form, preventing the formation of ammonia as well as its loss by volatilization, or the loss of the fertilizer value of the slurry.
The total ammoniacal nitrogen of the slurry (sum of NH4+ and NH3) can be in the range of 1.6 - 7.9 mg N/L, being 55 - 90 % of the total nitrogen, depending on the physiological state of the animals and the age of the slurry. Therefore, the value of slurry as a nitrogen fertilizer is closely related to the ammoniacal nitrogen content, which is lost during its time in storage in the form of NH3 if no technique is used to prevent this.
A distinction is made between total Kjeldahl nitrogen (which is the sum of ammoniacal and organic nitrogen) and total nitrogen, which also includes nitrates and nitrites. Excreted slurry contains only Kjeldahl nitrogen but, depending on the time spent in storage tanks open to the atmosphere, it may also contain small concentrations of nitrates and nitrites.
Acidification consists of the controlled addition of an acid, usually sulfuric acid, to maintain the pH of the slurry between 5.5 and 6, thereby maintaining virtually all ammoniacal nitrogen in its ionized form and preventing its volatilization. Figure 2 shows automatic acid dosing equipment on a pig farm in Denmark and Figure 3 shows acidification equipment attached to the tractor-tank to prevent ammonium loss during fertilization.

Figure 2. Acid storage tank on a pig farm in Denmark, that acidifies the slurry in the pits of the barn and outside storage in a controlled and automatic way.

Figure 3. Equipment attached to the tractor-tank for slurry acidification during agricultural application. Photo courtesy of www.vogelsang.info.
Acidification, apart from reducing ammonia emissions, has also been shown to reduce methane emissions to the atmosphere by limiting the growth of anaerobic microorganisms in the acidic environment.
The acidification system has been implemented to a significant degree in Denmark, where the storage time is 9 months and where regulations require high levels of efficiency in nitrogen fertilization with manure, for which it is essential to avoid the loss of ammoniacal nitrogen, especially when long storage times are necessary. In this sense, it is a suitable process in a situation where there is no nitrogen surplus, to take advantage of all the fertilizing potential of the slurry and to prevent emissions to the atmosphere.
Acidification involves an investment and operating cost, which can be partly offset by the reduction of chemical fertilizer use by utilizing all the ammonium in the slurry.
Acidification is also necessary in thermal concentration processes, when the aim is to take advantage of a thermal energy source to evaporate part of the water in the slurry and obtain a product of smaller volume to facilitate its transport, especially in a situation where there is a surplus. In this case, it is appropriate to use acidification on the liquid fraction of the slurry after an anaerobic digestion process, to prevent its volatilization and to reduce or minimize the content of volatile fatty acids, which tend to volatilize at an acidic pH, which would lead to organic matter contamination of the evaporation vapors or their condensate.

What role does acidification play in a non-surplus situation? 








