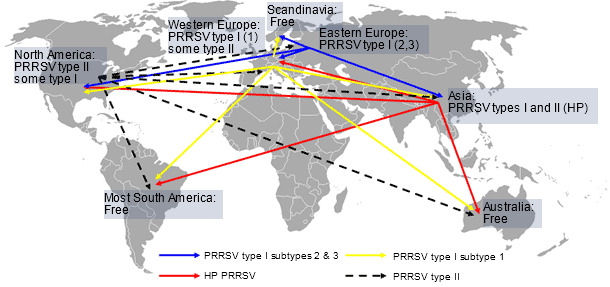
A recent study conducted by Iowa State University revealed that porcine reproductive and respiratory syndrome (PRRS) still causes major economic losses to pork producers in the US. Investigators estimated that PRRS costs $664 million annually or $1.8 million per day (Holtkamp D, et al. 2011). Reports suggest that the impact of the disease in Canada, Mexico, Asia and Eastern Europe appears to be similar. Since the disease emerged, more than 20 years ago, both PRRS virus type I and type II have disseminated geographically, gained genetic diversity and probably increased virulence (Murtaugh MP, et al. 2010; Li B, et al. 2006). In Western Europe though, the economic impact of PRRS is still in debate with extreme reports from fairly severe outbreaks to subclinical cases. A lower severity of the disease in Europe might be due to the relatively high prevalence of infection, the dominating farrow to feeder/finish production model and the predominance of type I isolates that despite a wide genetic diversity (Stadejek T, et al. 2008) induce less severe respiratory disease than type II isolates (Martinez-Lobo FJ, et al. 2011). Regardless of that interesting discussion, the risk of introduction of new PRRS virus type I or II isolates to high pig density areas with mostly farrow to finish farms in Western Europe is certainly present. Furthermore, the risk of intercontinental transmission of the highly pathogenic (HP) PRRS virus isolates detected in Asia is also real. At the same time, in other parts of the world such as Australia, most of South America and Scandinavia, the introduction of any PRRS virus could be catastrophic for the local industries (figure 1). This global turmoil indicates that PRRS virus itself as well as the risk and potential consequences of its transmission between regions should not be underestimated.
Figure 1. PRRS virus global distribution and hypothetical intercontinental transmission

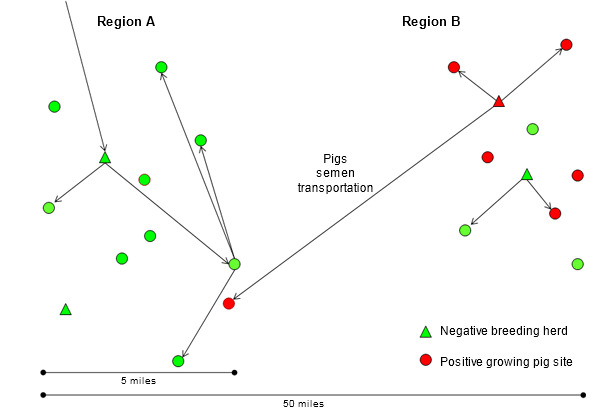
In the absence of a validated methodology for grouping PRRS virus isolates and for the purpose of this summary, a “new” isolate will be considered to be at least 3% heterologous (different) in the open reading frame (ORF) 5 nucleotide sequence to any isolate previously detected in the system (company) or region in the last 3 years. A “resident” isolate will be just the opposite. In several cases this arbitrary threshold can be meaningless and only used as a reference value. The rapid spread of PRRS virus over long distances in the 90’s and the relatively geographical confinement of resident isolates in recent years, when more sensitive monitoring protocols are used, suggest that new PRRS virus isolates need live pigs, semen or contaminated transport vehicles to be transmitted between regions (figure 2). As a hypothesis, new PRRS virus isolates originate from the continuous change of the virus in regions with constant transmission within and between herds (region B in figure 2). In countries where genetic multipliers and boar studs are intended to be PRRS negative, where significant progress has been made to quarantine and semen distribution protocols, the most common causes of long distance transmission are the movement of PRRS positive weaned or feeder pigs and the use of contaminated transport vehicles (region A in figure 2). Unfortunately, the distribution of PRRS positive replacement stock and semen continues to occur in several countries. Considering that because a region, system or herd has been already infected with one PRRS virus isolate can accept PRRS positive semen or replacement pigs is simply dangerous. A more virulent isolate can always be introduced and devastate production.
Figure 2. Introduction of a new PRRS virus isolate from region B to region A
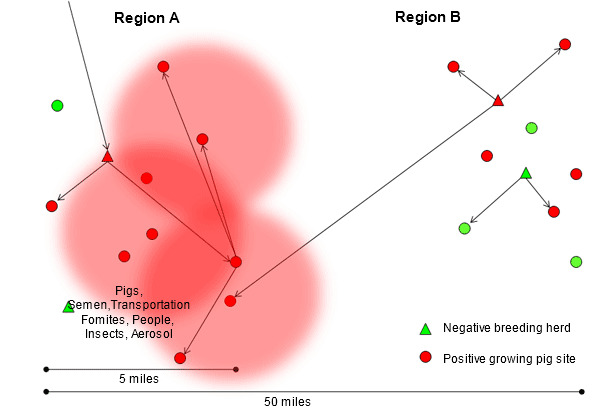
The research that Drs. Scott Dee and Satoshi Otake have conducted demonstrates that PRRS virus can be transmitted between herds by contaminated transport vehicles (Dee SA, et al. 2004), fomites (Otake S, et al. 2002), people (Otake S, et al. 2002), insects (Otake S, et al. 2004), manure and aerosol (Dee SA, et al. 2005). Transmission through these indirect routes is likely to occur over relatively short distances, from the first growing pig site infected with the new isolate (figure 2), in areas of high pig density probably exacerbated by poor biosecurity practices. Usually, when the first breeding herd becomes infected, several downstream sites receive acutely infected pigs turning each one of these groups into amplifiers of this particular PRRS virus isolate (region A in figure 3). Little can be done at this time to stop or slow down the epidemic. Interestingly, among the numerous genetically distinct isolates that are identified in high pig density areas every year, only a few are able to be transmitted between herds and even less able to widely spread and dominate the entire region or system. The factors that determine which PRRS virus isolates successfully disseminate and persist in geographical areas or pig flows are still to be fully investigated; however, it could be hypothesized that the ability of the isolate to efficiently replicate in pigs, its capability to be transmitted through multiple routes including aerosol and the existence of pig populations able to sustain the infection and then shed significant amounts of virus for a period of time could all be involved in this phenomenon.
Figure 3. Dissemination of the new PRRS virus isolate through region A
Controlled studies indicate that susceptible pigs that are exposed to live wild-type PRRS virus can shed for 70 to 100 days (Cano JP, et al. 2007; Linhares D, et al. 2012). The large populations of PRRS negative pigs serve as amplifiers of the virus in the recently infected region and represent a significant risk to neighboring farms for a long time. Same studies reveal that the use of modified-live virus vaccines in those recently infected populations reduces the duration of shedding by about 30 days. It is also expected that populations that have developed immunity against live PRRS virus will shed a heterologous virus (new introduction) at a lower amount and during less time than first time exposed or fully susceptible populations. Although the negative growing pig populations amplify the new isolates, it is the endemically infected continuous flow finishing sites or farrow to feeder/finish herds the reservoir of PRRS virus isolates in the region. This is because if growing pigs are managed in an all-in/all-out approach the new isolate will most likely be eliminated from that site within a few months. The results observed in pilot regional control initiatives suggest that the principle of exposure-immunity homogenization that has been successfully applied at the herd level to control PRRS virus represents a viable alternative to control PRRS virus at the regional level too.
In summary, the challenge that PRRS imposes to regions or companies could be overcome with the understanding of the epidemiology of the virus and the combination of strategies aiming to:
- Prevent new introductions through the consistent implementation of a comprehensive biosecurity program.
- Reduce the source of new isolates by attempting the elimination of PRRS virus from endemically infected and continuous flow populations.
- Contain the spread of new isolates by maximizing herd immunity, practicing strategic biosecurity and employing integrated surveillance to track the virus.
- Minimize the economical impact of the disease by intentionally managing exposure and immunity through air filtration, biosecurity, vaccination and pig flow modifications.