Assays available:
Polymerase chain reaction (PCR)

- Detects presence of specific sequence of viral nucleic acid (RNA)
- Sample types: serum or tissues
- Pros:
- Most sensitive and preferred method for detecting agent
- Can often do pooling of samples to lower cost while minimizing loss of sensitivity.
- Cons:
- Moderate cost
- Requires proper primers
Fluorescent antibody test (FAT)
- Detects presence of virus
- Sample types: tissues, especially tonsils
- Pros:
- Good sensitivity
- Cons:
- Cannot differentiate vaccine from wildtype virus
- Modified live vaccines can result in positive FAT for about 2 weeks
- Not feasible for large number of samples
- Results impacted by virus isolate used for assay
- Reliability is highly dependent on technician skills
- Cannot differentiate vaccine from wildtype virus
Monoclonal antibodies for differential immunoperoxidase test (MAbs)
- Detects presence of agent
- Sample type: FAT-positive tonsil or other organ
- Uses a series of three different monoclonal antibodies (MAbs) to help differentiate vaccine vs wildtype infection vs other pestiviruses
- Pros:
- Help differentiate vaccine vs wildtype infection vs other pestiviruses
- Cons:
- Moderate to high cost
- Requires a FAT-positive tissue sample
- Requires to be impacted by the three separate MAbs and one polyclonal antibodies (PAbs) used for the assay
- MAbs that recognize all CSF virus isolates
- MAbs that recognizes all CSF vaccine isolates used in that country
- MAbs that recognize bovine viral diarrhea (BVD) and borders disease (BD) viruses
- PAbs that recognize CSF
- Not feasible for large number of samples
- Reliability is highly dependent on technician skills
Enzyme-linked immunosorbent assay (ELISA)
- Detects presence of antibodies
- Sample types: serum
- Pros:
- Animals remain positive for several weeks
- Can be used in chronic cases
- Can be used to differentiate exposure to wild-type from gene-deleted vaccine
- Any positive result to wild-type virus is considered significant
- Cons:
- If possible, need to match the proper assay with the proper gene-deletion in the vaccine (usually E2)
- Takes at least 21 days for animals to become seropositive
- Can have cross-reactivity with other pestiviruses
Virus neutralization (VN)
- Detects presence of antibodies
- Sample types: serum
- Pros:
- Animals remain positive for several weeks
- Can be used in chronic cases
- Higher sensitivity than ELISA (can detect lower concentration of antibodies)
- Cons:
- Not feasible for large number of samples
- Takes at least 21 days for animals to become seropositive
- Unable to differentiate vaccine vs. wild-type infection
- Reliability is highly dependent on technician skills
- Reactivity is dependent on virus isolate used
- Can have cross-reactivity with other pestiviruses
Result interpretation:
PCR
- Positive: Wildtype or vaccine virus is present
- Negative: Negative or virus could have been missed if testing occurs late after infection
FAT
- Positive: Wildtype or vaccine virus is present
- Negative: Negative or virus could have been missed if testing occurs late after infection
MAbs immunoperoxidase test
- Positive: See Table 1
- Negative: Negative or virus could have been missed if testing occurs late after infection
Table 1. Monoclonal antibodies for differential immunoperoxidase test (MAbs) results interpretation. Source: OIE
| Polyclonal antibody | Monoclonal antibody specific for | Interpretation | ||
|---|---|---|---|---|
| CSF strain | CSF vaccine strain | BVD/BD strain | ||
| + | + | - | - |
CSF field strain |
| + | + | + | - | CSF vaccine strain |
| + | - | - | + | BVD/BV strain |
| + | - | - | - | Other non-CSF Pestivirus* |
* The existence of novel strains of CSF should always be considered and any isolate from cases where CSF is still suspected should be sent to an OIE Reference Laboratory.
ELISA
- Positive: Maternal antibodies or past exposure (>21 days) to wild-type virus or vaccine
- Negative: Negative or exposure to vaccine or wild-type virus too early to detect
Table 2. Helps demonstrate the DIVA (Differentiate Vaccinated from Infected) capabilities of some ELISA assays when using a gene deleted vaccine.

| ELISA non-differential | ELISA-E2 result | Interpretation |
|---|---|---|
| Positive | Positive | Exposed to wild-type virus |
| Positive | Negative | Vaccinated animal not exposed to wild-type virus |
| Negative | Positive | Assay error, result not possible |
| Negative | Negative | Not vaccinated and not exposed to wild-type virus |
VN
- Positive: Maternal antibodies or past exposure (>21 days) to wild-type virus or vaccine
- Negative: Negative or exposure to vaccine or wild-type virus too early to detect
Scenarios
Important to note that some countries may require approval by federal authorities before any testing for CSF can be done.
Suspected acute CSF outbreak in any age pigs and herd not vaccinated for CSF
- Collect whole blood from multiple affected animals and test via PCR; sample pooling may be considered
- Collect spleens and tonsils from dead pigs and test via PCR; sample pooling may be considered
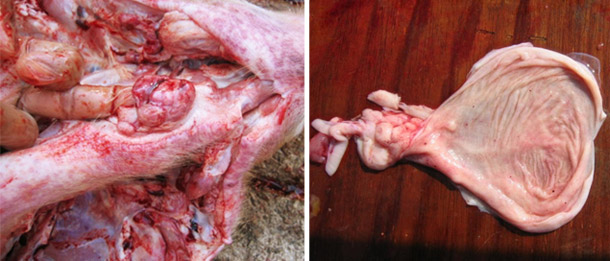
Suspected acute CSF outbreak in any age pigs and herd vaccinated for CSF
- Collect whole blood from multiple affected animals that have not been vaccinated for CSF within past 2 weeks and test via PCR; sample pooling may be considered
- Collect spleens and tonsils from dead pigs and test via PCR; sample pooling may be considered
- If PCR positive test tissue via FAT and MAbs immunoperoxidase test
Suspicion of chronic CSF circulation in any age pigs with no mortalities and herd is not vaccinated for CSF
- Collect whole blood from 30 pigs with clinical signs (targeted sampling) or randomly sampled (no clinical signs) and test via PCR (can pool samples if allowed) and tested individually with nondifferential ELISA
Suspicion of chronic CSF circulation in any age pigs with no mortalities and herd is vaccinated for CSF
- Collect whole blood from 30 pigs (not vaccinated within past 2 weeks) with clinical signs (targeted sampling) or randomly sampled (no clinical signs) and test via PCR (can pool samples if allowed) and tested individually with nondifferential ELISA and E2 differential ELISA