Assays available:
Gross pathology

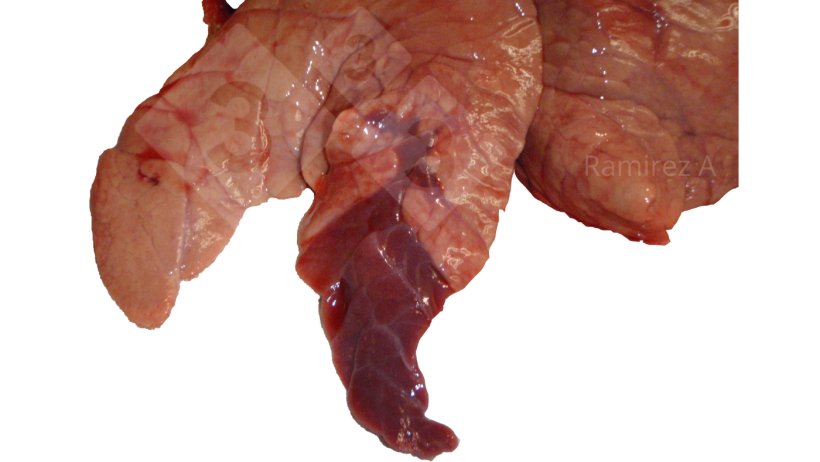
- Visual inspection of lung lesions
- Pros:
- Easy to do
- Can evaluate late infections in pigs sent to slaughter
- Cons:
- Not diagnostic/pathognomonic; many other possible causes
- Requires other diagnostic confirmation
Bacterial culture
- Isolation of live organism from tissues with lesions or bronchial alveolar lavage
- Sample types: Lung or bronchial alveolar lavage
- Pros:
- Traditional gold standard
- Pigs can test positive for 7-8 months (long period of colonization)
- Cons:
- Requires special media (Friis media)
- Available only in very few labs
- Expensive
- Very difficult to grow (many false negatives)
Immunohistochemistry (IHC)
- Detects presence of bacterial antigen
- Sample types: Lung tissue
- Pros:
- Detects bacteria at site of lesion (good proof of causation)
- Pigs can test positive for 7-8 months (long period of colonization)
- Cons:
- Correct tissue sample must be submitted – lung tissue must contain cross section of airways (bronchial epithelial cells)
- Requires significantly more bacteria to be present than PCR
- Only evaluating a small tissues sample
Fluorescent antibody (FA)
- Detects presence of bacterial antigen
- Sample types: Lung tissue
- Pros:
- Detects bacteria at site of lesion (good proof of causation)
- Pigs can test positive for 7-8 months (long period of colonization)
- Cons:
- Correct tissue sample must be submitted – lung tissue must contain cross section of airways (bronchial epithelial cells). See Figure 2 above.
- Requires significantly more bacteria to be present than PCR
- Only evaluating a small tissues sample
Polymerase chain reaction (PCR)
- Detects presence of specific sequence of bacterial nucleic acid (DNA)
- Sample types: Lung, swabs (nasal, laryngeal, tracheal-bronchial, or bronchial), or bronchial alveolar lavage
- Pros:
- Very high sensitivity (can detect small amounts of bacteria)
- Can do pooling of multiple samples to increase diagnostic sensitivity when making a herd diagnosis
- Pigs can test positive for 7-8 months (long period of colonization)
- Moderate cost
- Cons:
- Does not prove disease
- Oral fluids are a poor sample type (many false negatives)
Table 1: Schematic summary of research and field results on sensitivity of different sample types for Mycoplasma hyopneumoniae PCR testing.
| Relative Sensitivity | Pooling* | |
|---|---|---|
| Oral fluids | - | No |
| Nasal swab | + | Variable |
| Laryngeal swab | ++ | Beneficial |
| Tracheal-bronchial swab | ++ | Beneficial |
| Bronchial swab | +++ | Beneficial |
| Bronchial alveolar lavage | ++ | No |
| Lung tissue | ++++ | Yes |
* Pooling can be beneficial in increasing diagnostic sensitivity when making a herd diagnosis; not individual pig
Enzyme-linked immunosorbent assay (ELISA)
- Detects presence of antibodies
- Sample types: serum
- Pros:
- Animals can remain positive for a long time
- Can be used in chronic cases
- Cons:
- Specific antibodies detected and timing of detection may vary significantly between the many different commercial kits available
- Unable to differentiate maternal antibodies vs exposure
- Antibody levels do not correlate with protection
- Vaccination does not induce seroconversion (highly dependent on vaccine and assay used)
- Many infected pigs do not seroconvert
- Antibodies are not always detected for the entire duration of the infection
- Some cross reactivity with other Mycoplasmas especially M. flocculare which is non-pathogenic and commonly found in pigs
Result interpretation:
Gross pathology:
- Positive: Lung gross pathology can be used for possible diagnosis
- Negative: Early cases usually don’t manifest with extensive lung lesions
Bacterial culture:
- Positive: Confirmatory of disease
- Negative: Negative, bacteria could have been missed if testing occurs too late after infection or after antibiotic treatment, or simply unable to grow due to other bacterial overgrowth (present but very difficult to culture)
IHC

- Positive: Bacteria is present at site of lesion
- Negative: Negative, or bacteria could have been missed if testing occurs too late after infection or after antibiotic treatment
FA
- Positive: Bacteria is present at site of lesion
- Negative: Negative, or bacteria could have been missed if testing occurs too late after infection or after antibiotic treatment
PCR
- Positive: Bacteria is present; does not confirm disease
- Negative: Negative, or bacteria could have been missed if testing occurs too late after infection or after antibiotic treatment
ELISA
- Positive: Maternal antibodies or past exposure (usually 3 to 8 weeks post exposure) to vaccine or wildtype bacteria
- Negative: Negative or infection not stimulating seroconversion or too early to detect (usually takes 3 to 8 weeks post exposure)
Scenarios
Finishing pigs with chronic cough:
- Necropsy of 1-3 recently dead pigs or euthanize coughing pigs. Grossly evaluate lungs for cranial ventral consolidation. Collect multiple pieces of lung (making sure to include airways on sample) and place in formalin for IHC or FA evaluation.
- Percentage of lung with lesions is directly related to the economic impact of the disease.
Confirming Mycoplasma hyopneumoniae negative replacement animals
- Ensure there are no clinical signs of coughing.
- Collect at least 30 serum random samples from replacement animals and test via ELISA.
Monitoring replacement animals for shedding before introducing to a positive or negative breeding herd
- Ensure there are no clinical signs of coughing.
- Collect at least 30 random laryngeal swab samples from replacement animals. Pool in groups of 5 or 6 and test via PCR.