Assays available

Polymerase chain reaction (PCR)
- Detects presence of specific sequence of viral nucleic acid (RNA).
- Sample types: tissues, whole blood, serum, oral fluids, etc.
- Pros:
- Separate primers are used to detect both PRRS Type 1 (European) and Type 2 (North American) in the same sample at the same time.
- Very high sensitivity (can detect small amounts of virus).
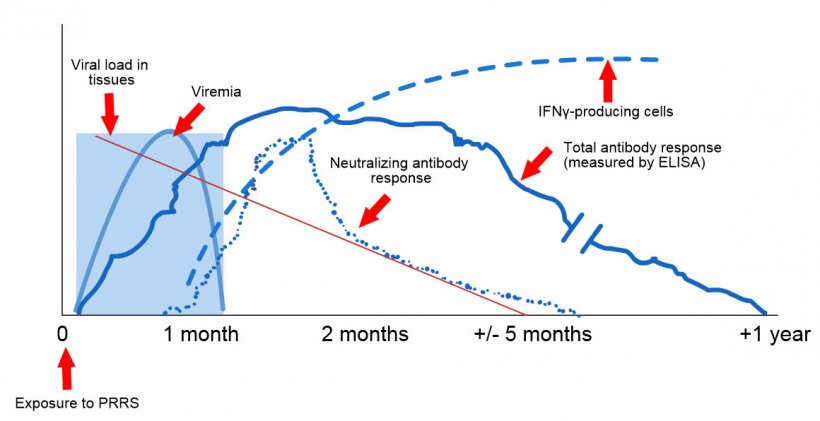
- Early detection - acute cases should be positive.
- Many different sample types can be used (tissue, blood, serum, oral fluids, etc).
- Moderate cost:
- Can often do pooling of 5 serum or tissue samples to lower cost while minimizing loss of sensitivity.
- Often do not pool oral fluids due expected higher Ct values (lower virus concentrations) which can result in significant loss of sensitivity.
- Cons:
- Laboratory must update primers periodically to avoid false negatives.
- Both PRRS Type 1 and Type 2 primers must be updated.
- Sequencing needed to differentiate vaccine virus vs. wildtype infection.
- Laboratory must update primers periodically to avoid false negatives.
Enzyme-linked immunosorbent assay (ELISA)
- Detects presence of antibodies.
- Sample types: serum or oral fluids (some kits).
- Pros:
- Most detect antibodies for both PRRS Type 1 and Type 2.
- Animals remain positive for several months (3-12 months).
- Can be used in chronic cases.
- Cons:
- Specific antibodies detected and timing of detection may vary slightly between the different commercial kits available.
- Takes 7 to 10 days for animals to become seropositive.
- Unable to differentiate maternal antibodies vs exposure.
- Unable to differentiate vaccine vs. wildtype infection.
Immunohistochemistry (IHC)
- Detects presence of viral antigen.
- Sample types: tissues.
- Pros:
- Detects virus at site of lesion (good proof of causation).
- Can identify low vs moderate vs high amounts of virus present.
- Cons:
- Correct tissue sample must be submitted.
- Requires significantly more virus to be present than PCR.
- Only evaluating a small tissues sample.
Genetic Sequencing
- Sequences virus’s genetic nucleic acids (RNA).
- Sample types: tissues, whole blood, serum, oral fluids, etc.
- Pros:
- Can differentiate wild type virus from vaccines.
- Can help differentiate new virus introduction from existing or past viruses.
- Cons:
- Expensive.
- Often only sequence ORF5 which is 600 out of ~15,000 base pairs.
- Samples with high CT values > 34 tend to be more difficult to sequence.
Table 1: Iowa State University Veterinary Diagnostic Laboratory sequence success based on PRRS PCR cycle threshold (Ct) values from oral fluid samples. Table from Chris Rademacher et al. 2016.
| Specimen | PCR Ct range | Total samples tested | Number samples sequenced | % Samples positive sequenced |
|---|---|---|---|---|
| All samples | <30 | 2016 | 2013 | 99.85 |
| 30.00-31.99 | 389 | 361 | 92.80 | |
| 32.00-33.99 | 324 | 265 | 81.79 | |
| 34.00-35.99 | 185 | 109 | 58.92 | |
| 36.00-37.00 | 65 | 26 | 40.00 |
Indirect fluorescent antibody (IFA)
- Detects presence of antibodies.
- Sample types: serum.
- Pros:
- Along with a PCR test, can serve as a confirmatory test for unexpected ELISA positive samples.

Diagram demonstrating the use of PRRS IFA as a confirmatory test for samples unexpectedly testing PRRS ELISA positive samples. A suspected negative sample that test ELISA negative is considered negative. If this sample unexpectedly test positive then a PRRS IFA can be done as a confirmatory test. That is if the IFA test is positive it is confirmed the sample is positive. If the IFA test is negative we would then assume it was a false positive as long as the PCR is also negative so as to confirm no recent infection.
- Along with a PCR test, can serve as a confirmatory test for unexpected ELISA positive samples.
- Cons:
- Not feasible for large number of samples.
- Results impacted by virus isolate used for assay.
- Reliability is highly dependent on technician skills.
Result interpretation
PCR
- Positive – Virus is present/circulating highly suggestive of causation especially with lower Ct values and clinical signs are present. Recent vaccination with a modified live virus can result in positive PCR results.
- Negative – Negative or Virus could have been missed if testing occurs late after infection.
ELISA
- Positive – Maternal antibodies or past exposure (usually > 7-10 days post exposure) to vaccine or wildtype virus.
- Negative – Negative or Infection too early to detect (usually must be at least 7-10 days post exposure).
IHC
- Positive – Virus is present at site of lesion.
- Negative – Negative or virus could have been missed if testing occurs late after infection.
Genetic Sequencing
- Vaccine virus – Expect > 99% homology.
- Wildtype virus – Estimate about a 1-2% loss of homology per year.
IFA
- Positive – Maternal antibodies or past exposure (> 7-10 days post exposure) to vaccine or wildtype virus.
- Negative – Negative to vaccine or wildtype virus or infection too early to detect (must be at least 7-10 days post exposure).
Scenarios

Sow/Gilt abortions
- Aborted fetuses: Collect 6-8 fetuses and pool samples for PCR testing. Only about 50% of fetuses aborted will be PCR positive (so need to sample many fetuses), but those that are positive will have a large concentration of virus and therefore can pool in groups of 10 for PCR testing.
- Aborted sows/gilts: Collect serum from recently aborted (< 10 days) sows/gilts for PCR testing. Can pool in groups of 5. ELISA testing is not useful as it usually will take 7-9 days for naïve sows/gilts to test positive.
Sow/Gilts reproductive problems
- Collect 15 to 20 samples from affected and 15 to 20 samples from non-affected (30 – 40 samples total) gilts/sows and test via PCR (pools of 5 or 6) and ELISA (individually).
Weak piglets in farrowing
- Can collect family oral fluids from several litters with weak pigs and test via PCR.
- Can collect testicles (if castrated), tails, tongue tissue (dead pigs) from pigs from different litters in the farrrowing room. Large number of samples can be pooled together for testing.
- Collect serum samples from 10 affected litters by collecting 2 to 3 piglets per litter and test via PCR on pools of 5 or 6 piglets per pool. Ensure piglets have not been vaccinated for PRRS.
Growing pig with acute clinical signs of PRRS
- Collect oral fluids from 4 to 6 different pens and test individually via PCR. Do not pool samples for testing.
- Collect 15 to 30 serum samples from pigs with clinical signs or random sampling and test via PCR. Can pool in groups of 5 or 6 for PCR testing.
Growing pig with chronic clinical signs of PRRS
- Collect oral fluids from 4 to 6 different pens and test individually via PCR. Do not pool samples for testing. Can also test oral fluids samples via ELISA.
- Collect 30 serum samples from pigs with clinical signs or random sampling and test via PCR. Can pool in groups of 5 or 6 for PCR testing. Also test individual samples via ELISA to confirm exposure.