Enzyme-linked immunosorbent assay (ELISA)
ELISA is an extensively used assay for quantitative detection of antibodies against pathogens in biological fluids and has high specificity and sensitivity. It is performed in 96-well microtiter plates and employs the antigen-antibody interaction and enzyme-dependent development reaction (colorimetric, fluorescent or chemiluminescent). The signal measured as optical density (OD) by a spectrophotometer can be directly or inversely proportional to the concentration/amount of antibodies detected.

In order to detect total anti-viral or anti-bacterial/mycoplasma antibodies, an indirect ELISA is employed in which a pathogen antigen is bound on the solid phase (plate well) and the sample is incubated to form an antigen-antibody complex. Then, a conjugated secondary antibody is added to generate the readout signal. This system allows many samples to be screened with a single conjugated secondary antibody.
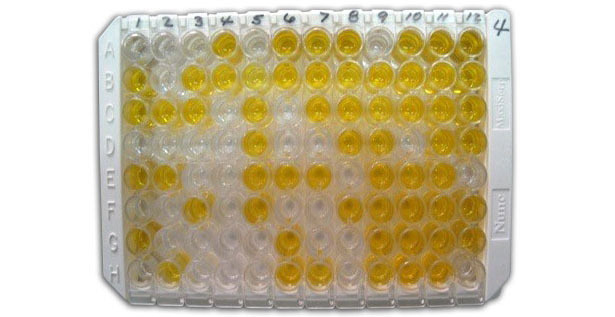
The competitive and inhibition ELISA quantify antibodies in the sample based on their ability to interfere with a pre-titrated assay. In both assays, a pre-titrated system is challenged with the addition of the sample in which the antibody binding activity is determined from the degree of interference.
For instance, in order to detect the humoral response to PRRSV, the N protein of the virus is used to capture total PRRSV-specific antibodies onto antigen-coated 96-well plates, and a peroxidase-conjugated secondary antibody is used to visualize captured anti-PRRSV antibodies.
The humoral immune responses are variable among animals and levels of antibodies detected do not necessarily reflect the virulence of the PRRSV isolate. Furthermore, maternally derived antibodies (MDA) can interfere, leading to false positive results. Generally, ELISA antibodies allow detecting total antibodies which are the pool of circulating plasma cells and pathogen-specific memory B cells, therefore in order to properly detect the memory humoral response, detection of virus-neutralizing antibodies directed against immunodominant epitopes is fundamental. Total antibody ELISA detection can however provide useful information on the seroconversion and boosting effect upon subsequent vaccinations and/or infection.
Serum virus neutralization (SVN) test
The SVN test allows detecting and quantifying virus-specific neutralizing antibodies in serum. A two-fold dilution of the serum sample and a known quantity of virus are usually pre-incubated and then added to target cells sensitive to viral infection allowing not neutralized virus to infect the cells and determine the highest dilution which can neutralize the virus infection, and the presence of a cytopathic effect.
The SVN assay is highly sensitive and specific, measure the titer of neutralizing antibodies post-infection or after vaccination. Titer quantification can be based on the presence or the absence of the cytopathic effect or the evidence of viral infection using an immunoreactive technique. The SVN test is useful to evaluate the level of serological cross-reactivity between vaccine antisera and virus isolates in order to assess and correlate with cross-protection.
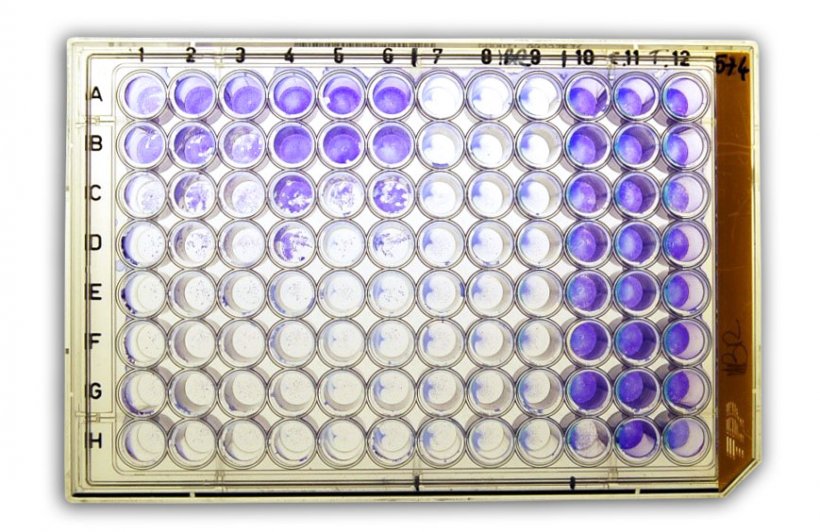
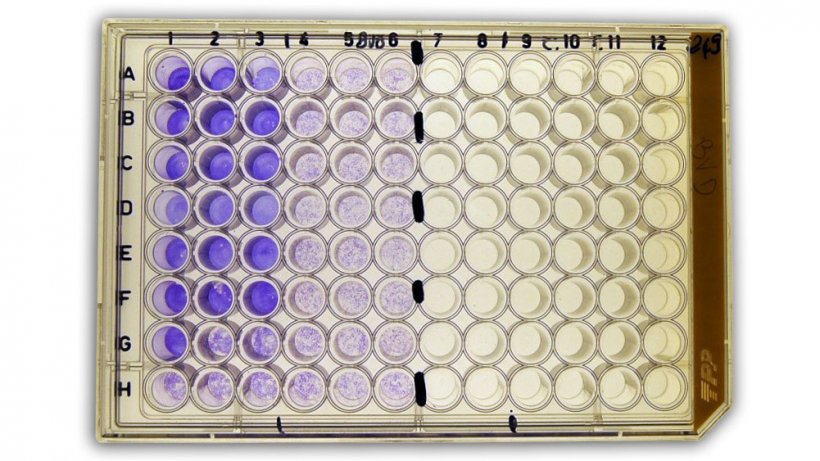
Haemoagglutination (HI) test
HI is a serological test for the detection of antibodies and works on the principle that the haemagglutinin of influenza viruses, like that of other viruses, has a haemagglutinating effect on red blood cells, therefore it is the standard method used for the detection of anti-influenza antibodies. This means that blood will clot on addition of these viruses, because the red blood cells bind to the haemagglutinin on the virus surface.

Specific antibodies induced as humoral immune response to infection or vaccination with the virus can inhibit the agglutination by binding to the haemagglutinins.
If the sample contains specific antibodies to the haemagglutinin, addition of the influenza virus produces an antigen/antibody reaction. This is made visible by the subsequent addition of erythrocytes. These are no longer agglutinated because the reaction is inhibited.
Serum titration can be used to determine the dilutions at which haemagglutination is still inhibited. The reciprocal value of the highest dilution at which inhibition of agglutination can still be observed provides the antibody titer.
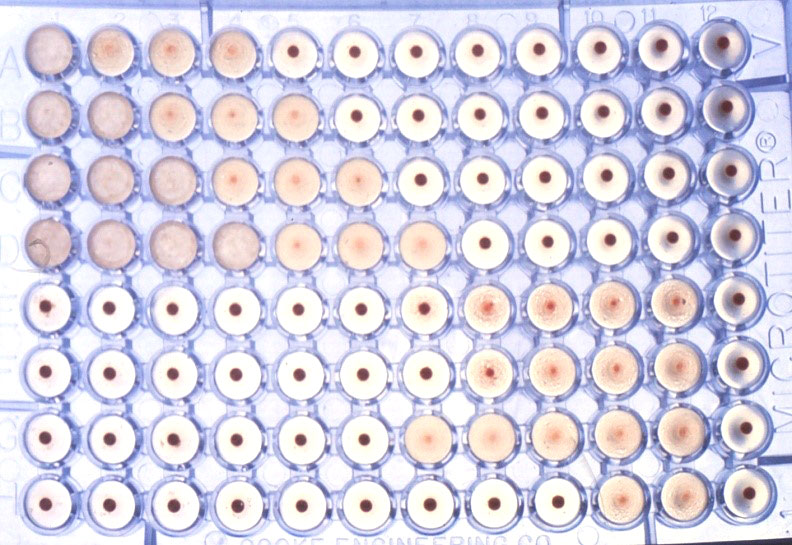
Immunoperoxidase monolayer assay (IPMA)
The IPMA is widely used for detection of antibodies against a virus, especially those viruses that do not display cytopathic effect such as PCV2. Neutralizing antibodies (NA) are the main antibodies responsible for protection and clearance of infection. Since there is a positive correlation between IPMA antibody titers and VNA titers, IPMA titers might be considered an indirect measure of NA titers. IPMA is not the assay of choice for routine diagnostics, as it depends on virus infected cell cultures, and is relatively time-consuming for screening many serum samples. For these reasons, replacement of IPMA by more automatic assays using an objective end-point readout, such as ELISA, is often employed.






