Osteochondrosis (OC) is a degenerative and non-infectious condition that affects joints and that has a hereditary component. The pathogenesis starts with ischemic necrosis of the cartilage that progresses towards an abnormal ossification and possible fractures of the articular cartilage. We could say that there is a chance for reducing the initial cause (80% prevalence between 8 and 12 weeks of age) or the progression (with a 60-70% of recovery at 25 weeks of age). Nevertheless, the aetiology is not clear (Olstad et al., 2015), and it could include traumas or collagen fragility (Laverty and Girard, 2013; Finnøy et al., 2017). Also, in order to reduce the prevalence of OC, we should make use of genetic selection, although this could be difficult, initially, because the lean tissue growth potential is genetically associated with OC (Aasmundstad et al., 2013). On the other hand, other factors that could have an influence on the progression of the disease are not well known.
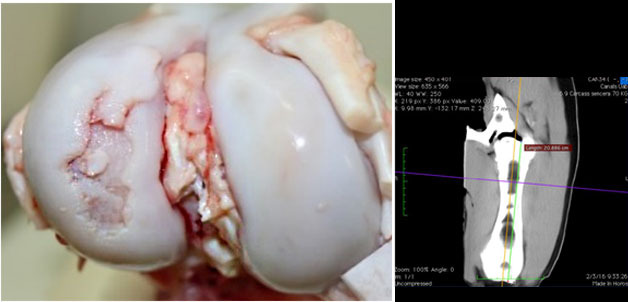
Macroscopic view of a knee joint with a severe osteochondrosis lesion in the lateral femoral condyle.

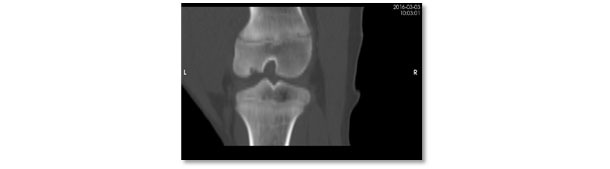
Computerised tomography view of the same lesion. Severe osteochondrosis lesion with lack of ossification in the subarticular region of the lateral femoral condyle.
The use, in the diet, of minerals, trace minerals and some functional components (e.g. vitamins) can modify the bone composition and structure (Riet et al., 2013). Nevertheless, their importance in pathological processes is controversial. In the case of bone development, calcium (Ca) and phosphorus (P) are very important. In the diet, a minimum digestible Ca:P ratio is needed (1:1), and it varies depending on the P level (e.g. 1.25:1) if P meets the recommendations (50-80 kg LW) NRC (2012). Also, Ca recommendations to maximise mineralisation are greater than for growth (<1.35:1; Lagos et al., 2018). With a P deficiency, the growth rate and bone mineralisation are reduced, but without effects on OC nevertheless.
Sulphur is a mineral that is needed for cartilage resistance, and it takes part in the proteoglycans-hyaluronic acid binding (Myllyharju, 2014). It also has therapeutic effects on joint lesions (Ouattara et al., 2016). In the case of gilts, supplementation with methionine (1,1:1 methionine: lysine) as a source of highly available sulphur was associated to a drop in OC (Frantz et al., 2008). Likewise, and in the case of magnesium, Counotte et al. (2014) reported improvements in OC in foals supplemented with 4 g/d from 5 to12 months of of age.
Trace minerals (TM) are also essential elements. Nevertheless, there are significant differences between growth requirements and practical recommendations. In fact, some suggest greater requirements for bone and hoof development than for growth (e.g. turkeys, Ferket et al., 2009; and pigs, Barneveld and Vandepeer, 2008). However, more practical evidences are needed. Zinc (Zn) interacts with several components of the bone extracellular matrix (e.g. osteoblasts, metaloproteinases and growth factors). Thus, the increase in Zn in the diet increases the ashes content and bone resistance linearly (Veum et al., 2009). Similarly, silicon maximises collagen production. By supplementing pigs (1,000 mg silicon/kg feed), reductions in OC have been seen (Frantz et al., 2008). On the contrary, although manganese participates in the synthesis of proteoglycans, it does not affect OC. Copper (Cu) is apparently more important, and it is a catalyst of Cu-lysil oxidase, providing resistance to collagen. The combined supplementation of Cu (250 mg/kg) and Mn (100 mg/kg) improved the biomechanical properties of the cartilage and reduced OC (Frantz et al., 2008). Conversely, Tóth et al. (2016) used inorganic + organic TM sources (150 + 50 g/kg Zn, 50 + 20 mg/kg Cu, and 16.5 + 10 mg/kg Mn) without seeing apparent effects on OC at an early age (12 weeks; 100% OC) or at late age (24 weeks, 1.5% OC prevalence). Therefore, all the basal levels in their treatment seemed enough to reduce the progression of OC. On the other hand, a similar TM supplementation decreased the incidence of lameness and improved bone quality (Fabà et al., 2018; 2019). These conflicting results suggest other more practical hypotheses: Can the potential of the TM interact with the inherent or mechanical risk for suffering OC? Or, on the contrary, are there interactions between the minerals or other components of the diet (this is, antimicrobials, ZnO, phytate, etc.) that can cause temporary deficiencies that affect OC? In fact, a Zn excess can displace copper metallothionein and the copper-dependent lysil oxidase enzyme, this resulting in the abnormal production of cartilage and bone. This fact is critical on the prestarter stage, this coinciding with the emergence of OC. Nevertheless, these interactions require therapeutical or greater ZnO levels (Hill et al., 1983).
Other deficiencies, such as vitamin (e.g. B folic acid) deficiencies, can have negative consequences on bone development. The most important deficiency is that of vitamin D, that reduces Ca and P availability, and affects bone metabolism and other physiological functions (Clarke, 2008). Nevertheless, vitamin A, B, C, D and E supplements do not have an influence on OC (Nakano et al., 1987, Ytrehus et al., 2007).
High levels of sugars increase the insulin and insulin-like growth factor peaks. These effects can compromise the chondrocytic activity, although they have a wide spectrum and it has not been proved that they increase OC in pigs. Other supplements such as glucosamine sulphate or glycosaminoglycans (e.g. chondroitin sulphate), or omega-3 fatty acids have been suggested for improving the synthesis of proteoglycans and cartilage, although no effects have been seen on the OC of gilts (Neil et al., 2005; Frantz et al., 2008).
It is clear that nutritional deficiencies reduce bone quality and can have an influence on OC. But the conflict between results questions the potential of nutritional supplements on OC. More research is needed to understand its pathogenesis and progression, specially including the interactions at a practical level with growth rate, genetics and management.





