The economic success and sustainability of swine production is tightly linked to feed efficiency. One of the most effective ways to improve feed efficiency is to increase the lean growth of pigs. Improvements in defining the nutritional requirements of pigs and genetic selection for improved composition of growth have allowed for dramatic improvements in feed efficiency over the past twenty years. With the development of technologies to examine global gene expression changes, interest in the interactions between nutrition and genetics has increased. Nutritional genomics, also known as nutrigenomics, studies both genotypic specific nutrient requirements as well as interactions between nutrients and genes. Understanding the complex interactions between nutrition and genetics that underpin feed efficiency is crucial for identifying novel interventions to improve the sustainability of swine production. Application of nutrigenomics in young pigs is especially important, as pre-weaning growth and growth throughout the nursery are highly predictive of lifetime growth performance (Main et al., 2004).

Nutrigenomics and growth in swine
The development of muscle, fat and bone tissue are intertwined and dynamically regulated by nutrition, occurring through changes in gene transcription, protein synthesis, and epigenetic regulation. Over 40% of lean body weight is made up of skeletal muscle tissue whose growth is determined by the rate of protein synthesis and degradation (Rehfeldt et al., 2004). Adipose tissue distributes all over the body and serves as an important tissue for energy storage. Throughout life bone is constantly undergoing deposition and reabsorption. Nutritional programing regulates the growth of these tissues by turning genes "on" and "off". These epigenetic modifications can be passed from generation to generation. Current research has indicated that cell proliferation, differentiation and programmed cell death are all under control of small ribonucleic acids called microRNA (Bengestrate et al., 2011). Understanding the changes of messenger RNA and microRNA profiles in pigs fed with different diets can reveal the major epigenetic markers regulating pig growth. New techniques involving global gene expression analysis could shed light on these critical events that are likely governed by some key RNAs. Understanding these underlying mechanisms of nutrient-mediated regulation of bone, fat, and muscle growth are crucial for swine production improvement.
Muscle Satellite Cells
Muscles contain a self-renewing pool of precursor cells that are responsible for the life time muscle growth of the animal. These satellite cells reside just outside of the muscle fibers, and under the right conditions, proliferate and differentiate into the growing muscle. Muscle growth and size are determined by the number (hyperplasia) or size (hypertrophy) of the muscle fibers. Post-natal muscle growth is a hypertrophic event and skeletal muscle fiber number is set at birth; however, pre-weaning nutritional deficiencies could lead to the interruption of elongation of existing myotubes resulting in lower fiber numbers (Rehfedlt et al., 2004). Even slight restrictions (70%) in dietary phosphate had an impact on satellite cell proliferation and activity in neonatal piglets (Figure 1), as well as those piglets who were fed supra-adequate phosphate diets also had a higher feed conversion efficiency (Alexander et al., 2012). The genes governing the proliferation and differentiation potential of satellite cells are perfect markers for determining the complex interplay that nutrients have on lifetime lean muscle growth. Several candidate microRNAs have been identified as epigenetic markers and regulator of satellite cell programming. These and other works demonstrate the need and importance of neonatal nutrigenomics research in the swine industry.
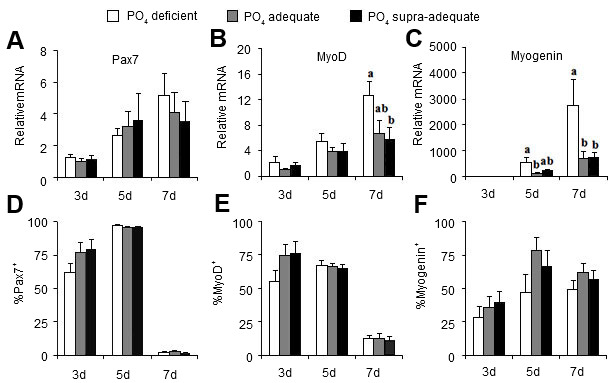
Figure 1. Dietary phosphate treatment effects on genes governing satellite cell proliferation (Pax7) and differenation (MyoD, Myogenin). Relative gene expression (top panels) and in vitro protein (bottom panels) (Alexander et al., 2012).
Mesenchymal Stem Cells (MSCs)
Pre-weaning growth is also partially governed by mesenchymal stem cell (MSC) populations. A MSC possesses the ability for self-renewal and multipotentiality. Under proper conditions, MSCs can be differentiated to bone, cartilage, and fat tissue as well as nonmesodermal cells such as neurons, hepatocytes, pancreatic islet cells, and endothelial cells (Chen et al., 2004; Oswald et al., 2004; Pournasr et al., 2011; Tzeng et al., 2015). Lineage allocation of MSCs is under tight regulation of nutritional signals. Manipulation of MSC lineage allocation through nutritional programing becomes an innovative and important avenue for promoting lifetime growth performance in pigs. Dietary supplementation has been shown to regulate MSC populations and activities. For instance, dihydroxy-cholecalciferol [1,25(OH)2D3], a hormone critical for bone development and animal production, stimulated porcine MSCs to differentiate towards a fat cell lineage (Mahajan and Stahl, 2009) (Figure 1). Similarly, dietary calcium level also has great effects on MSC activity and lineage allocation. Excess dietary calcium supplementation significantly increased MSC proliferation and promoted MSC differentiation towards adipogenesis over osteogenesis (Li and Stahl, 2014).
These studies indicate that neonatal mineral and vitamin supplementation have a huge impact on bone development and body composition via the regulation of MSC and satellite cell programing. Future efforts will focus on using nutrigenomics to identify and optimize nutrient components in order to achieve greater swine production with desired meat quality.
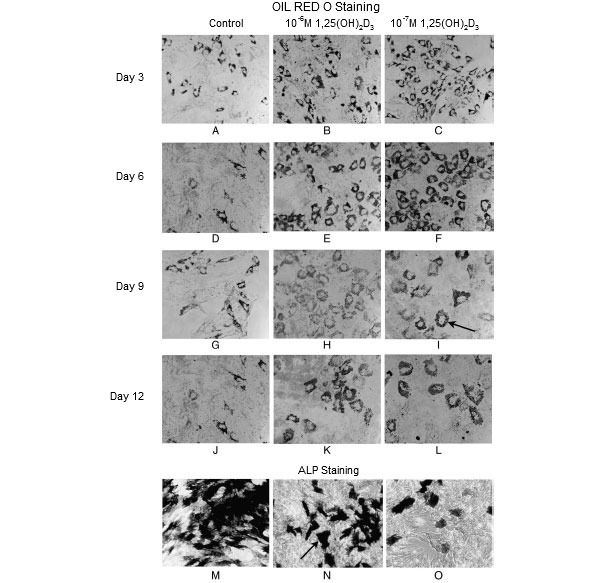

Figure 1. Oil red O staining for neutral lipids in fat cells and alkaline phosphatase staining (ALP) in osteoblast-like cells (Mahajan and Stahl, 2009). Dihydroxy-cholecalciferol [1,25(OH)2D3] promoted mesenchymal stem cell adipogenesis at 3 days (A-C), 9 days (G-I), and 12 days (J-L) post-differentiation. However, it inhibited mesenchymal stem cell osetogenesis (M-O).


