Porcine circovirus type 2 (PCV2) is the aetiological agent of a number of diseases that may affect pigs of all productive ages; PCV2 systemic disease (PCV2-SD) and PCV2 subclinical infection (PCV2-SI) are the most relevant ones. This group of diseases has been collectively named porcine circovirus diseases (PCVD). The wide range of pathologies associated to this virus in combination with its worldwide distribution results in a relevant economic impact in swine production industry. In this article it will be discussed the properties of PCV2 and their implications on pathogenicity, transmission and persistence.
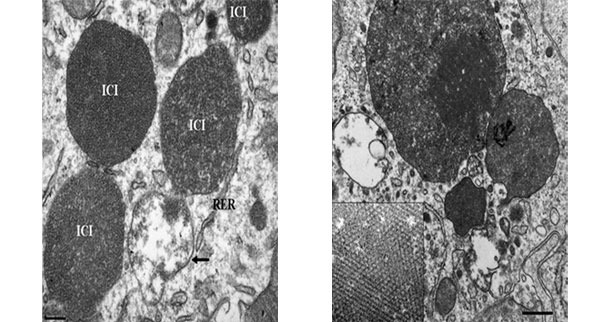

Electron micrograph of a lymph node from a PCV2-SD affected pig. Note the presence of PCV2 intracytoplasmic inclusion bodies (ICI); in some areas, the viral particles can be arranged in paracristalline arrays (inset of the right image). Picture courtesy of Carolina Rodríguez-Cariño, PhD thesis.
PCV2 is a non-enveloped, icosahedral virus with single stranded circular 1.76-1.77 kb DNA that belongs to the genus Circovirus from the family Circoviridae. Its size is of about 12-23 nm in diameter, being the smallest known virus that affects mammals. PCV2 genome is composed by 11 putative open reading frames (ORFs), but protein expression has been only described for ORF1 (Rep gene, encoding for nonstructural replicase proteins, Rep and Rep’), ORF 2 (Cap gene, encoding for the capsid protein) and ORF 3 (encoding for a non-structural protein that induces apoptosis in PK-15 cells). The capsid protein has immunogenic properties, being able to induce an effective humoral and cellular response; not surprisingly, it is the common element in commercial PCV2 vaccines.
Phylogenetic studies have revealed a high (>93%) nucleotide identity between PCV2 strains. Despite several studies carried out in an early stage did not consider significant sequence differences between PCV2 isolates, subsequent works divided PCV2 strains into two main groups designed as genotype "a" (PCV2a) and "b" (PCV2b). Later, a third genotype (PCV2c) was retrospectively described in pigs from Denmark in the1980’s. Finally, a fourth genotype (PCV2d) has been recently described in China, although nowadays is known to be present also in other parts of the world. Under appropriate circumstances, PCV2a and PCV2b are able to experimentally reproduce PCV2-SD. However, PCV2b has been linked to disease expression to a higher frequency through experimental infections and by epidemiological studies, where the shift of the predominant genotype from PCV2a to PCV2b in the livestock has been associated with the epidemic outcome of PCV2-SD. Under this situation, it must not be discarded the possibility of a concurrent infection of pigs by more than one genotype at a farm level. Nowadays all commercial vaccines against PCV2 are based on PCV2a, although they have demonstrated excellent results evidencing cross-protection against PCV2b. Going a step beyond, experimental PCV2b vaccines have shown even better results against PCV2b infection than PCV2a ones, suggesting an apparent more effective homologous protection.
PCV2 biological and physicochemical properties have not been completely characterized. The virus is highly resistant in the environment, showing also high resistance to chemical and thermal treatments (Table 1).
Table 1. Effect of environmental conditions and chemical and thermal treatments on PCV2 infectivity.
| Effect on PCV2 infectivity | |
| pH |
|
| Thermal treatments |
|
| Chemical treatments |
|
PCV2 is a swine specific virus that can infect domestic and wild boars. The ability of PCV2 to infect other species such as bovine, ovine, caprine, equine, canine, feline, rabbits, guinea pigs, poultry or humans has been investigated, showing no apparent susceptibility. Other studies point that PCV2 is able to replicate and transmit between mice; in this sense, it has been suggested a role of mice and rats as alternate host or mechanical vectors in swine herds, where PCV2 has been found in these species in pig farms, but not outside them. However, the role of mice in the whole PCV2 epidemiological picture is probably of low importance. PCV2 has been detected in all tested excretion/secretion routes, being present in nasal cavity, tonsil, bronchi, saliva, ocular conjunctiva, faeces, urine, milk and semen. Transmission usually occurs by the oro-nasal route but it is also possible transplacentary. Infection is maintained in the herd by sows, which are the origin of infection for piglets; and these latter ones may experience a long lasting infection, with viraemias that may last even until 28 weeks of age. Under this situation, where PCV2 shows a high resistance in the environment and high capabilities of transmissibility, it is not surprising that PCV2 is a persistent virus with a ubiquitous distribution, present in practically all locations where pigs are reared.










