Introduction to the gut microbiota
The gastrointestinal tract (GIT) of the pig is home to a diverse and abundant population of bacteria, archaea, fungi and viruses, collectively known as the microbiota. The majority of microbial members in this population are described as symbiotic, as they benefit the host in diverse ways, such as increasing energy harvested from the diet, detoxification of xenobiotics, provision of essential nutrients, exclusion of pathogens and programming of the immune system. However, until very recently swine industry practices and research has focused on the small percentage of microbiota that represent pathogens. It is time we focus on the microbiota as a source of disease resistance rather than as a source of disease.

Immune development and disease resistance
Colonization of the GIT of animals by microorganisms post birth from the surrounding environment is vital for the proper development and function of the immune system. A healthy microbiota not only outcompetes pathogens for nutrients and space, but regulates host immune function helping the host protect itself form both bacterial and viral infection. Germ-free animals have an underdeveloped immune system, increasing the risk of disease pathogenesis due to improper immune system priming (Macpherson et al., 2004). While germ-free animals are not realistic, it is clear that not all microbes are equal in their ability to regulate the immune response and protect the host from disease. A study in conventionally raised mice has been performed where the transfer of microbiota from animals resistant to enteric infection to animals lethally susceptible was sufficient to provide infection resistance (Willing et al., 2011). While similar studies have not been performed in pigs it has been demonstrated that the microbiota of pigs that will shed high and low levels of Salmonella can be differentiated prior to infection (Bearson et al., 2013). Furthermore, rearing piglets in a clean environment with and without exposure to an adult microbiota resulted in changes in immune development as well as the ability to tolerate infection with Mycoplasma hyopneumoniae (Schachtschneider et al., 2013).
Acquiring the right microbes
As a result of co-evolution, gut microbes benefit by protecting their host from disease. To emphasize the importance of host specificity and co-evolution, it was shown that strains of a ubiquitous vertebrate gut symbiont, Lactobacillus reuteri, group into distinct clades for human, poultry, pig and rodent (Spinler et al., 2014). Importantly, immune system development is much less robust in response to colonization by microbes from a different animal species (Chung et al., 2012). In the human population there has been a growing recognition of a “disappearing microbiome” due to changes in our behaviour, particularly surrounding hygiene and antibiotic use, resulting in increased incidence of immune mediated diseases (Martínez et al., 2015; Blaser et al., 2009). Similar to the heightened hygienic practices in the Western world, intensive livestock systems use vigorous hygienic and biosecurity measures to minimize pathogen exposure. One recent study compared the microbiome of domestic to wild pigs and indeed found differences in community composition, particularly with respect to Bifidobacterium (Ushida et al., 2015). How can we expect our livestock to acquire a healthy microbiome when there is such limited exposure to the microbes that they evolved with?
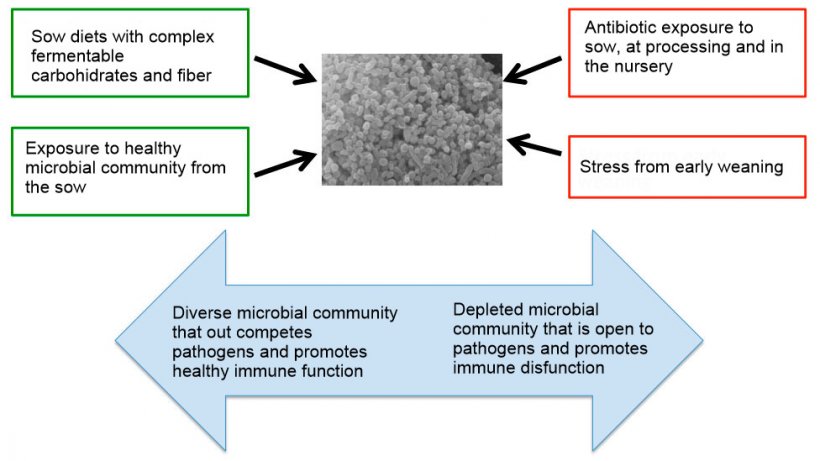
Raising piglets in excessive hygiene conditions promotes innate immune activation, suggesting impaired immune homeostasis (Mulder et al., 2011). Furthermore, rearing pigs in more ‘natural’ outdoor conditions increases the proportions of symbiotic Lactobacillus spp. vs. indoor-reared pigs, which harboured more potentially pathogenic phylotypes of Proteobacteria (Mulder et al., 2011; Mulder et al., 2009). As depicted in Figure 1, hygiene used in intensive rearing systems for pigs may reduce the ability for a stable microbiota to form, impeding normal immune development and function, potentially increasing risk of pathogenic disease (Schmidt et al., 2011). In addition to exposure to the right microbes, research has shown that development of a stable microbial community in pigs requires continuous microbial exposure during early life (Schmidt et al., 2011). While finding strategies to increase exposure of health promoting co-evolved microbes, it is important to recognize that biosecurity remains a critical issue in swine production.

The consequences of antibiotics: beyond antibiotic resistance
Antibiotics have been used as a key tool to support animal health in intensive livestock production systems. While antibiotics are used to remove and target pathogens, they represent one of several factors impacting the development of a healthy microbiota (Figure 2) and can sometimes support pathogen colonization. Antibiotic treatments in experimental models have revealed that microbes are needed for maintenance of innate mucosal defenses (Willing et al., 2011). Therapeutic doses of antibiotics have been shown to reduce host expression of antimicrobial peptides and mucin genes, which are essential in preventing pathogens from reaching the mucosal surface (Menendez et al., 1013; Wlodarska et al., 2011). While the impact of antibiotics on pig innate defense has been minimally studied, anecdotally, farmers find increased disease subsequent to a therapeutic antibiotic treatment. It should also be noted that antibiotics can have long-term effects on the composition and function of the microbiota (Janczyk et al., 2007). I have heard of producers that will go grab a handful of dirt to throw in the pen when piglets are scouring. It is also a common practice to administer probiotics after a therapeutic course of antibiotics. These producers are on the right track, however, efforts to develop more targeted interventions is needed. While there are still many questions that need to be answered, the growing research effort to identify the populations of microbes that are going to best support the pig are underway.