Much effort has been attempted to control PRRSV infection. The initial attempts were based on the use of strategies known to be effective for controlling the other diseases in swine, such as segregated early weaning; however, results were inconsistent. The central component of PRRSV-control is the reduction of the spread of the virus within the breeding herd, thereby preventing the infection of offspring prior to weaning.
The presence of subpopulations of exposed and non-exposed sows in chronically PRRSV-infected breeding herds assists in the maintenance of virus circulation in the breeding herd over time. In this regard, proper management of replacement stock is essential to reduce/eliminate formation of subpopulation and the spread of the virus in the breeding herd.
If internal replacement stock is available, the herd can be ideally closed for 4-6 months to stabilize the breeding herd (herd closure). During the period of herd closure, no naïve animals are added into the positive breeding herd so that seronegative subpopulations are eliminated, therefore virus circulation is expected to cease in the breeding herd.

Figure 2: Proper management of the gilt pool is an important component of a successful PRRS control program.
In order to accelerate the reduction of seronegative subpopulations, procedures to expose replacement stock may be carried out prior to herd closure. If replacement stock needs to be introduced from off-site herds, acclimatization including exposure and isolation has been used to allow the introduction of immune and non-shedding animals into PRRSV-positive breeding herd. It is commonly suggested that acclimatization take place in separate facilities specifically designated for this purpose (gilt developer). In order to accommodate the need for extended acclimatization period, it is also recommended that larger group of younger animals (weaning age-pigs) be introduced less frequently throughout the year. As the first process of acclimatization, exposure takes place for 1-2 months to insure that all animals develop viremia and seroconversion against exposing PRRSV strain. Exposure can be achieved by various ways including commingling with cull sows or nursery pigs demonstrating PRRSV-clinical signs, oral exposure of feed back materials including serum/tissue homogenates from clinically ill pigs obtained from previous acute outbreak of PRRSV in the herd, intramusclar injection of serum collected from PRRSV-viremic pigs, or vaccination by commercial products. A period of 3-4 months following the exposure allows the exposed animals to recover from acute phase of PRRSV-infection and develop protective immunity against PRRSV. Therefore, the exposed animals following the proper recovery period of time are unlikely to shed the virus when they are introduced into the breeding herd.
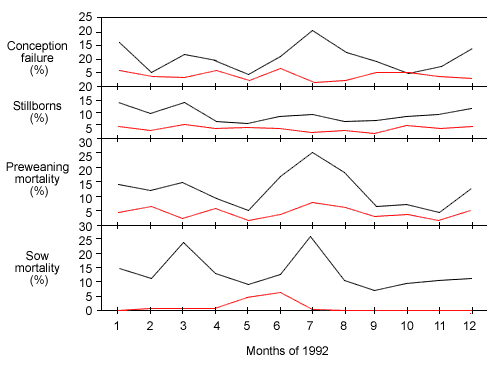
Figure 1: Production data from 2 herds with different gilt introduction strategies. The white line represents a farm introducing PRRSV-naïve gilts directly into the breeding herd in the absence of gilt development. In contrast, the red line represents a farm introducing gilts at 25 kg, allowing for exposure to farm-specific strains of PRRSV during the grow-finish period.
Vaccination
Commercial vaccines against PRRSV can be used as an aid in reducing the clinical consequences of infection. Currently, both killed and modified live vaccines for PRRSV are commercially available for administration to sows and pigs; however, protective immunity may not be complete against all forms of heterologous challenge. Both types of vaccines have their inherent advantages and disadvantages. Killed vaccines are known to be safer, particularly in pregnant sows and farm-specific autogenous killed products can be manufactured. However, killed vaccines require boosters, are expensive and do not induce cell mediated immunity which is essential for protection against PRRSV. In contrast, modified live vaccines replicate in the host and do induce a cell-mediated response; however, are not as safe as killed products and can be shed from vaccinated to non-vaccinated animals if used improperly. Recent evaluation of a commercially available PRRSV modified live vaccine indicated that use in a therapeutic manner, i.e. application in the face of an outbreak provided protection against heterologous challenge and prevented the shedding of wild-type virus to naïve sentinels co-mingled within the infected population. However, it was also shown that vaccination did not prevent infection of wild-type virus and that repeated vaccination did not eliminate wild-type virus from the body of the pig in the case of heterologous strains. In addition, proper on-farm management of vaccines is essential to enhance efficacy, such as proper refrigeration, avoidance of exposure to extreme temperatures, selection of proper needle length to insure intramuscular penetration and awareness of expiration dates.
Serum
The use of serum inoculation of sows and gilts for PRRS control is now widespread throughout North America . This method consists of the intramuscular injection of all incoming naïve replacement gilts or/and whole breeding herds with serum derived from acutely infected pigs which contains a farm-specific isolate of PRRSV. This method is based on the assumption that protection in infected pigs is complete following re-exposure to the homologous variant of PRRSV. Important aspects of this control strategy include sequencing of the virus, quantifying the level of PRRSV in a dose of serum and screening the inoculum to insure the absence of adventitious agents, such as PCV-2
Management
For control of PRRS at the farrowing house level, McREBEL is a proven technique. McREBEL PRRS management consists of several strategies to reduce the risk of virus spread prior to weaning. Examples of strategies within the McREBEL PRRS program include preventing the cross-fostering of piglets after 24 hours of life, humanely destroying PRRSV-infected pigs, needle management and strict all in-all out pig flow in the farrowing house. Application of these practices has been shown to improve the post-weaning performance of pigs due to the reduction in horizontal virus transmission prior to weaning.
Conclusions
In conclusion, each PRRS case requires careful assessment by the practitioner in order to determine which strategies are most likely to be cost-effective. The application of carefully collected diagnostic data in combination with a working knowledge of all available strategies has proven to be successful in many cases.





