PRRS diagnostics have evolved over the years to include several valuable testing options. The type of animals to sample and the nature of the sampling procedure depend on the scenario for which we complete the testing: Should we test sows, piglets, finishers, or all of them?; Are we performing a targeted sampling or a random sampling of the population?; and what should our sample size be? We also need to decide on the type of sample: Are we collecting serum, tissues, oral fluids, or semen? And finally, we cannot forget all of the questions related to the stage of the disease at the time of sampling. When did the clinical signs appear and do we expect the diagnostic test to detect virus, identify lesions, or detect antibodies? Finally, it is critical that we understand the advantages and the limitations for each diagnostic test.
Understanding the options and choosing the correct diagnostic strategy for each scenario for which we need to complete PRRS sampling will increase the effectiveness of any herd health management program.

Scenarios for which we complete PRRS sampling
The most obvious scenario is for disease investigation, when symptoms of a potential PRRS outbreak appear in a negative or PRRS stable farm. In this case, the test is performed by veterinarians in order to understand the cause of clinical signs and the origin of the pathogen causing the problems. Another very common scenario is when monitoring for the absence of PRRS in a previously positive and unstable herd that has undertaken a control or eradication strategy and wants to understand their progress. Finally, PRRS testing is extremely common for disease surveillance purposes. In this last scenario, testing is performed on negative farms and normally requires a greater number of samples in order to maximize the degree of confidence of the PRRS-free status of the population.
Our diagnostic goals guide our test selection
Historically, we have classified PRRS diagnostic tests into 3 types:
- Those that detect lesions: Post mortem observations and histopathology, which can only be performed at a laboratory under a microscope. Lesion detection tests are most commonly used early in a clinically obvious PRRS outbreaks;
- Those that detect the virus: polymerase chain reaction (PCR), virus isolation (VI), and immunohistochemistry (IHC) tests. Viral detection is the most reliable diagnostic tool for early confirmation of the presence of the virus;
- Those that detect antibodies: Enzyme Linked Immunosorbent Assay (ELISA), immunoperoxidase monolayer assay (IPMA), and indirect immunofluorescence assay (IFA). Immunological confirmation of PRRSV contact requires a longer period of detection, but confirms exposure when the virus cannot be detected.
Photo 1. Flat-bottomed, 96-well ELISA plate used for PRRSV serology. Positive samples are shown in blue. Source: Base Pair Biotechnologies.
The test characteristics determine the value of the test
It is very important to review the diagnostic test SENSITIVITY (SE) and SPECIFICITY (SP) when considering any test. Knowing this information helps to properly interpret and act upon the possible outcomes.
- SE is the ability of a diagnostic test to correctly identify true positive samples. A test with a low SE will yield too many false negative results.
- SP is the ability of a diagnostic test to correctly identify true negative samples. A test with a low SP will yield too many false positive results.
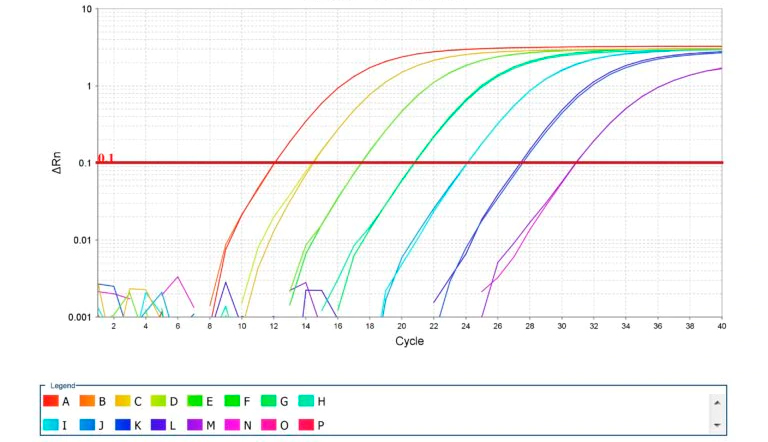
Photo 2. Amplification of standard curves for qPCR. Source: Thermo Fisher Scientific Inc.
Diagnostic options by PRRS scenario
All scenarios and diagnostic options are summarized in Table 1.
1. Outbreak investigation: For a farm experiencing an outbreak, the goal of the diagnostic protocol will be to confirm the infection and, if possible, to genetically characterize the strain. Early veterinary involvement in the outbreak will help to achieve these goals effectively. If pigs with classical clinical signs are present, targeted sampling of these animals to look for gross lesions is strongly recommended. After identifying characteristic lesions (i.e. heavy, non-collapsed lungs with marbled appearance) in the field, PCR and histopathology should be performed to confirm the diagnosis. Consequently, genetic sequencing should be completed to identify the strain. Sequencing is the key to understanding the epidemiology (i.e. origin, resident or emergent) of the potential new virus through comparison with other known strains.
2. Disease monitoring: In a PRRSV positive farm, in order to minimize the negative impact of the disease due to production instability, control programs (sometimes followed by eradication) should be implemented as soon as possible to produce negative piglets. The goals of the diagnostic program in this case will be to demonstrate key parameters that indicate PRRS stability (i.e. gilt acclimation, breeding herd immunity, and production of negative piglets). Serology is used to confirm good gilt and breeding herd exposure and PCR is used to confirm the absence of the virus in newborn and weaned piglets. Under these conditions, we utilize tests with high a SE (i.e. the fewest false negatives as possible). Because we are dealing with a farm where the expected prevalence of PRRS positive piglets is likely to be very low or absent, a large number of samples will be required to confirm the PRRS status with a high degree of confidence. Assuming the absence of clinically affected piglets, randomly selected piglets and newly introduced negative gilts within the farm are our best targeted populations for sampling. Most of the sample types (i.e. serum, oral fluids, processing fluids, and tissues) are of value but it is important to understand the SE and SP differences between tests when evaluating the outcomes. Pooling should always be considered when possible to make the analysis more affordable.
3. Disease surveillance: When performing disease surveillance on negative farms, we commonly select tests with the highest SP (i.e. the fewest false positive results) as possible. These farms (i.e. insemination centers or multipliers), on many occasions, are required to document their status on a routine basis. In the case of sow farms, ELISA test is the best option to show the absence of PRRS exposure. It is affordable, fast, and has a good SE and SP. It is common for these farms to run a secondary serological test to determine if their unexpected positives results (normally 1 to 2% of the total samples) are true or false positives. Indirect fluorescence antibody test (IFA) or immunoperoxidase monolayer assay (IPMA), both based on indirect staining of pre-prepared monolayers of infected cell, serve as common confirmatory tests for unexpected positive on ELISA. PCR as an early detection test is used on: 1) oral fluids prior to the delivery of breeding aged replacement gilts; and 2) serum or blood swabs from boars in insemination centers.

In summary, choosing the correct tests, at the correct time, in the correct animals, and interpreting them correctly will increase the speed, accuracy, and cost-effectiveness of our PRRS diagnostic strategies.
Table 1. Summary of scenarios and strategies for diagnostics
Outbreak investigation
- Farm PRRS status
- Unstable
- Active transmission of the virus
- New PRRS introduction
- High disease prevalence
- Diagnostic objectives
- Detection of infection
- PRRSV strain identification
- Animals
- Those with clinical signs
- Stillborns
- Types of sampling
- Targeted sampling
- Fewer number of animals
- Pooled samples
- Sample
- Tissue
- Serum
- Primary diagnostic option
- Necropsy to identify the lesion
- Pros
- “Very fast”
- On farm
- Inexpensive
- Cons
- Low SE/SP
- Secondary diagnostic option
- PCR/Virus sequencing to detect/identify the PRRSV strain
- Pros
- High SE/SP
- Fast, 24h
- Results can be quantified (RT-qPCR)
- Cons
- Potential false positives due to cross contamination: ensure good sample management (during sampling and processing)
Disease monitoring
- Farm PRRS status
- Stable
- Resident PRRSV
- Low disease prevalence
- Diagnostic objectives
- Monitoring stability / control
- Monitoring eradication programs
- Animals
- Piglets (newborn and weaned)
- Gilts
- Types of sampling
- Random sampling/High number of animals
- Targeted sampling/Lower number of animals
- Pooled samples
- Sample
- Oral fluids
- Serum
- Processing fluids
- Piglet tongue fluids
- Primary diagnostic option
- PCR to detect the antigen (RNA) from ≈7 days post-infection
- Pros
- High SE/SP
- Fast 24h
- Results can be quantified (RT-qPCR)
- Cons
- Potential false positives due to cross contamination: ensure good sample management (during sampling and processing)
- Secondary/Alternative diagnostic option
- ELISA to detect antibodies (IgM, IgG, N) from ≈14 days post-infection
- Pros
- Low cost per sample
- Results in 2-4 days
- Confirms lab history of lack of virus exposure
- Cons
- Lower SP
- Can not differentiate between vaccine and field virus exposure
- Results can not be quantified
Disease surveillance
- Farm PRRS status
- PRRS negative farm
- Diagnostic objectives
- Ensure negative status
- Animals
 |
|
4. Types of sampling
|
4. Types of sampling
|
|
5. Sample
|
5. Sample
|
|
6a. Primary diagnostic option (sow herd, gilts and piglets)
6b. Primary diagnostic option (breeding aged replacement gilts)
7. Secondary diagnostic option
|
6. Primary diagnostic option
|











