The immune response to infection by type 1 and type 2 PRRSV has similar outcomes. Namely, antigen-specific humoral and cell-mediated responses are elicited, antibodies are made to a range of viral proteins, the infection is resolved, and a memory response is generated that protects against future challenge. However, the response to the two different viral genotype families is quantitatively different in important aspects. Both the severity of infection and the intensity of immune responses are reduced in type 1 PRRSV infection as compared to type 2 PRRSV infection. As a result, the study of immune protection against re-infection has been difficult in the type 1 infection model since disease can be difficult to demonstrate reproducibly, and since infection can occur without causing disease. Thus, interpretations can be misleading in the absence of solid positive and negative control with reproducible endpoints. Because of this problem, the majority of research on protective immunity and vaccinology is based on type 2 PRRSV, in which clinical disease can be reproducibly demonstrated in both respiratory and reproductive models of PRRSV infection. This limitation is relevant since type 1 PRRSV is the most important virus form in Europe, whereas PRRS in Asia and North America is caused primarily by type 2 forms.
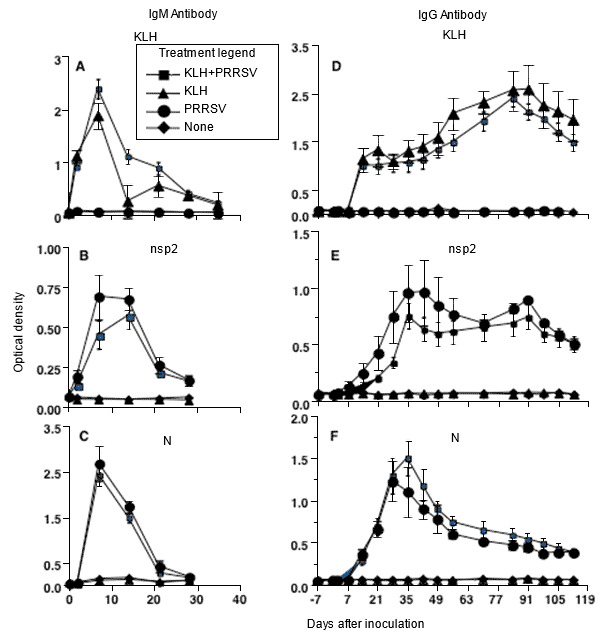

Figure 1. Antibody response kinetics to type 2 PRRSV infection, showing that response to individual PRRSV proteins is the same as to an unrelated protein antigen, keyhole limpet hemocyanin (KLH) (modified from Mulupuri et al. 2008. J. Virol. 82:358-370).
The first time naive pigs are infected with PRRSV, an innate immune response occurs with evidence of interferon induction by virulent viruses, but absence of interferon by infection with low-virulent viruses. Several viral proteins block the innate interferon response but, regardless of the effectiveness of innate immunity in countering PRRSV infection, a normal humoral immune response is observed. It includes rapid production of IgM antibodies to a range of viral proteins, followed by switching to IgG antibodies to the same proteins. The kinetics of response are identical to the response to an control, noninfectious protein antigen. PRRSV-specific antibody secreting plasma cells and memory B cells are present primarily in spleen and lymphoid tissues that drain lung and reproductive tissues. Tonsil is the primary site of memory B-cells. Neutralizing antibodies appear later in infection at about the time viremia ends, and are at a low level.
Induction of memory B- and T-cells is critical for virus-specific protection against a future infection, so are essential features of protective immunity. PRRSV-specific memory cells are readily produced, and respond to individual viral proteins in cell culture. However, challenge of immune pigs does not produce a classical anamnestic response. Thus, the immune resistance status cannot be predicted by looking at serological responses following challenge. Rather, protective efficacy must be determined by virological and clinical outcomes that assess reduction or prevention of infection and disease. Challenge studies consistently show that immunity induced by live vaccines and virulent viruses induce substantial protection against future infection by unrelated viruses. The conserved antigenic epitopes that are essential for heterologous protection have not been identified.
Various immunopathological consequences of PRRSV infection, including hypergammaglobulinemia and antibody-dependent enhancement of infection, have been reported in experiments; but evidence that they occur in the field is lacking. By contrast, immunosuppressive effects of viral infection are difficult to reproduce in experiments, but there is little question in the field that overall herd health declines following a PRRS outbreak.
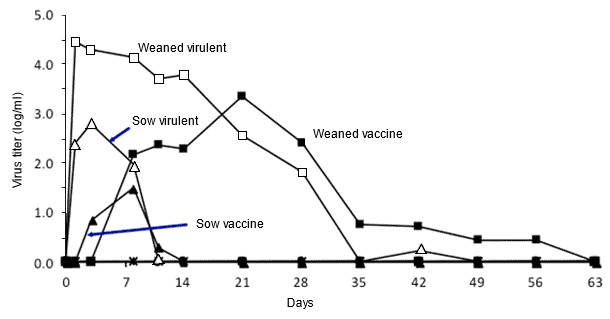
Figure 2. Effect of pig age on PRRSV growth. Quantitative viremia levels over time in weaned pigs and adult sows exposed to a type 2 virulent or attenuated PRRSV (adapted from Klinge et al. 2009. Virol J. 6:177-187).
Extensive variation is a hallmark of PRRS, both in the virus and in the pig’s response to the virus. Type 1 and type 2 PRRSV are genetically different, and within each group there is huge variation. Understanding the interaction of PRRSV with pigs is complicated by the fact that most research is performed with only a few virus isolates, many of which are not representative of isolates currently causing current disease. Similarly, there is tremendous variation in the response of naïve pigs to primary infection or immune pigs to field virus infection. For example, fattening pigs and adult sows have higher innate resistance to infection than do weaned pigs, although the immune response induced by infection appears to be equivalent at all ages. Resolving the basis of viral and host variation will require identification and characterization of key B- and T-cell epitopes conserved among diverse PRRSV, and of the molecular and structural details of key effector antibodies, T-cell antigen receptors, and MHC molecules that mediate broad, cross-protective immunity.