Farm introduction
This is a cooperative that has 2 piglet production units (PPU) and an artificial insemination center (AIC), located in the state of Paraná (Brazil). The PPUs are located at 60 km from each other and the AIC 35 km from PPU 1 and 60 km from PPU 2 (Figure 1).
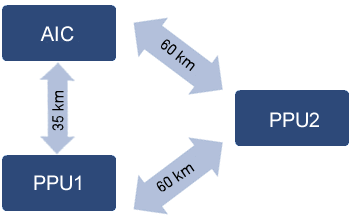

Figure 1. Distance between PPU farms and the AIC.
In March 2011 there is a report by the managers of the PPU’s which reflected an increase of returns in females (at 17 to 28 days, cyclic and acyclic, with a return rate of 22%) and mucous-like, of white to brown (chocolate) vulvovaginal discharge hours after artificial insemination. The highest incidence occurred in gilts but also affected multiparous sows. Weaned sows are housed in boxes with a controlled temperature of 21 ° C and relative humidity between 60-70%, and are kept in the same area for at least the first 7-8 weeks of gestation.
This problem occurred on two farms. For biosecurity measures first the AIC is visited and then the PPU’s, due to the protocols of the consulting firm. The first clinical suspicion’s arose with the presence of mycotoxins, so that the nutrition company took samples, monitored, and quantified them. They found nothing in the raw materials.
Health status
At the PPU’s, the animals are negative for seroconversion to PRRS and Aujeszky and positive for Mycoplasma hyopneumoniae. They have adopted appropriate biosecurity measures in their routines that allow for good disease control with a low incidence of clinical signs indicating the presence of acute or chronic diseases.
Replacement on commercial farms begins with the acquisition of weaned F1 females from a multiplier which has its own great-granparent and grantparent sows and the acquisition of genetic material, which meets the basis of genetic improvement, is produced by the purchase of great-grandparents' and grandparents' boar semen through a preset program. The company’s AIC produces 7,500 doses per month.
THE AIC
The AIC is a modern and semi-automated, and uses waste materials in their processes. Boars are free of antibodies for PRRS, Aujeszky, Leptospirosis, and Brucellosis and are vaccinated every 6 months against Erysipelas, Leptospirosis and Parvovirus. They are housed in 7.5 m pens with a regulated temperature of 21 ° C (range between maximum and minimum of 4-6 ° C), a relative humidity of 60-70% and 12-14 h of light.
The extraction room has a sump pit and lab units are adequately separated into clean, dirty and intermediate zones. The laboratory itself used water produced with reverse osmosisin order to produce doses. All equipment is stainless steel, single-use materials and used for the handling of semen. Doses are packaged automatically in plastic tubes.
Technical visit
During the visit to the AIC samples were taken of the water, pure and diluted semen, as well as materials that are part of the production and manipulation of semen doses, as shown in Table 1 with their respective results.
Table 1. Bacteriology.
| Sample | UFC / mL – Agent |
| Water recently processed through reverse osmosis | No growth |
| Water collected from the homogenizer with diluent | 36 CFU |
| Dipslide Water with diluent | Growth and isolation of Burkholderia cepacia |
| Water with diluent | Growth and isolation of Burkholderia cepacia |
| Homogenizer bag / Helix | Growth and isolation of Burkholderia cepacia |
| Collection tube - Blister | No growth |
| Container for the dispenser | No growth |
| Straw Bottle | 10 CFU |
| Dispenser nozzle | No growth |
(CFU) colony-forming unit

Table 2. Bacteriology in diluted semen
| Sample | Isolated agent |
| Diluted semen -1 | Burkholderia cepacia |
| Diluted semen– 2 | Burkholderia cepacia |
| Diluted semen– 3 | Burkholderia cepacia |
| Diluted semen– 4 | Burkholderia cepacia |
Results
Thus it was verified that there was a prior contamination in the homogenizer tank (in the helix and the disposable plastic bag lining of the tank). Contamination was also established in doses produced at that time due to high concentrations existing in the tank (plastic bag and propeller) being isolated in the same strain as the dilution tank.
1 st Conclusion: There was discarded plastic bag in the tank after each use being renewed just once a week. The recommendation is to change all disposable material after each dose production.
Farm visit (PPU 1)
During the visit to the PPU, samples were taken of the first urine, discarding the first drops and collecting only the middle portion in sterile containers and maintaining basic hygiene precautions throughout the procedure. The material was then immediately prepared and sent, refrigerated, for laboratory processing.
It was observed that 15% of urine specimens showed a bacterial growth of Burkholderia cepacia. This is a bacterium that is commonly found in soil or water and can live for long periods in humid environments. Some studies have linked the presence of Burkholderia cepacia to humans with cystic fibrosis present in respiratory diseases.
Also a gilt with an abundant presence of vulvovaginal secretions was selected that, in clinical evaluation, all parameters showed a normal physiological state with no indication of loss of appetite or fever (rectal temperature was 38.7 ° C). The only clinical observation was the presence of mucus and the presence of mucus threads and hyperemia in the vaginal mucosa. The sow had recently been inseminated (received the first dose of semen during the morning and proceeded to collect the sample at the end of the afternoon).
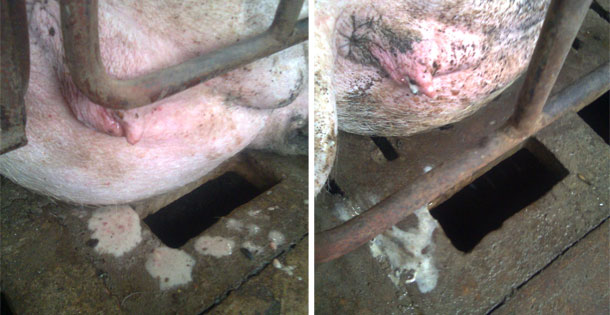
Figure 1. The images show vulvar secretions.
Swabs were taken of these secretions for bacterial isolation. The results are shown in the following table:
Table 3. Results of bacterial isolation.
| Sample | Isolated agent |
| Vaginal mucosa | Growth and isolation of Burkholderia cepacia |
| Cervix mucosa | Growth and isolation of Burkholderia cepacia |
| Uterus lining | Growth and isolation of Burkholderia cepacia |
| Bladder mucosa | No growth |
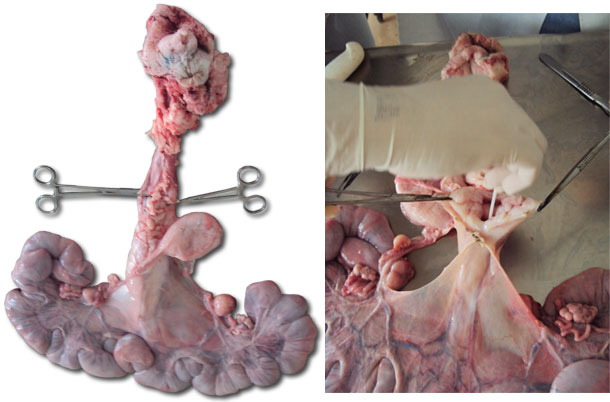
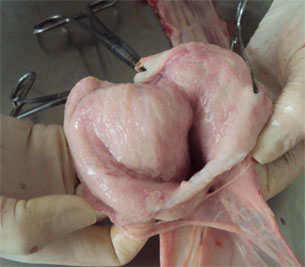
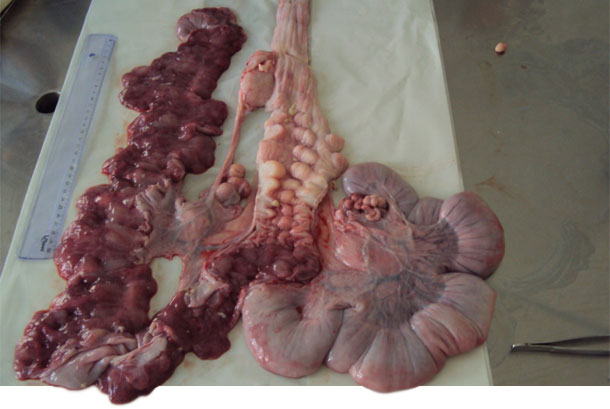
| Figure 2. Reproductive tract of a gilt. | Figure 3. Opening the cervix to collect secretions with a swab. |
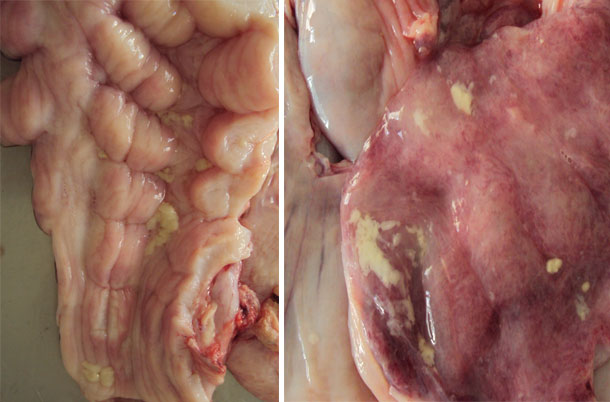
Figure 3. Presence of mucus in the lining of the cervix and the uterine lining.
|
|
Other newly inseminated females with the same clinical symptoms were selected for collection of swabs of vulvovaginal secretions by using disposable speculums for a better opening of the vaginal mucosa and deep insertion of the swab. We selected a nulliparous female with the same clinical signs and a complete insemination to be slaughtered and to be necropsied. We extracted the entire genital tract and, when opened, took samples of purulent mucus of the cervix, vagina and uterine horns for bacterial culture and isolation.
2 nd Conclusion: In all the material collected for bacterial isolation the same bacteria was detected: Burkholderia cepacia.
Management strategies that were adopted:
1. Resorting to medication using injectable ceftiofur for sows with problems;
2. Discard sows repeating with the presence of secretions;
3. Adopt the procedure of autoclaving all contaminated laboratory materials;
4. Use a disposable bag in the dilution tank every day.
5. Increase basic staff health care and laboratory collection, begin to use disposable masks during the preparation of semen doses;
6. Visits to the center and farms every 15 days, collecting material from all stages of semen production process until conditions return to normal;
7. Avoid storing semen doses for more than 72 hours;
8. The problem was solved in the lab three weeks, but losses were extended for 10 weeks.
The diluent that was used contained antibiotics, but bacteria were resistant, as shown in the antibiogram, and as shown in the following table. Also the addition of antimicrobial molecules sensitive to B. cepacia in diluents was requested.
Table 4. Antibiogram-RG 131
| Antibiotics |
Burkholderia cepacia (water with diluent) |
Burkholderia cepacia (semen diluted by 88645) |
||
| Inhibition zone | sensitivity | Inhibition zone | sensitivity | |
| Amoxicillin 10 mcg | 6 mm | R | 6 mm | R |
| Ampicillin 10 mcg | 6 mm | R | 6 mm | R |
| Apramycin 15 mcg | 16 mm | S | 16 mm | S |
| Ceftiofour 30 mcg | 26 mm | S | 26 mm | S |
| Colistin 10 mcg | 6 mm | R | 6 mm | R |
| Enrofloxacin 5 mcg | 26 mm | S | 26 mm | S |
| Spectinomycin + Lincomycin 100+9 mcg | 14 mm | R | 14 mm | R |
| Florfenicol 30 mcg | 16 mm | I | 16 mm | I |
| Gentamicin 10 mcg | 6 mm | R | 6 mm | R |
| Neomicin 30 mcg | 6 mm | R | 6 mm | R |
| Norfloxacin 10 mcg | >30 mm | S | >30 mm | S |
| Penicilin G 10 um | 6 mm | R | 6 mm | R |
| Sulfazotrim 25 mcg (Sulfametazin+Trimetropim) | 6 mm | R | 6 mm | R |
LEGEND: S = sensitive, R = Resistant, I = Intermediate
Method: Diffusion in agar
Reference: CLSI (Clinical And Laboratory Standards Institute)