Introduction
The farm was a commercial 600 sow birth to bacon unit in Ireland that increased to 1,000 sows within a year.

The vaccination protocol operated is illustrated in Table 1.
Table 1. Vaccination programme.
| Vaccine | Gilts | Sows | Boars | Piglets |
| Parvovirus | 2 ml injection @ selection | 2 ml injection 2 weeks post farrowing | 2 ml injection twice a year | |
| Erysipelas | 2 ml injection @ selection and 2 weeks pre service | 2 ml injection 2 weeks post farrowing | 2 ml injection twice a year | |
| Colibacillosis& Clostridiasis | 2 ml injection 6 and 2 weeks pre farrowing | 2 ml injection 2 weeks pre farrowing | ||
| PRRS (MLV) | 2 ml injection @ selection and @ 60 days post-service | 2 ml injection at 6 days post-farrowing and @ 60 days post-service | 2 ml injection twice a year | |
| PCV-2 | 1 ml injection at weaning | |||
| Enzootic pneumonia | 1 ml @ 10 - 14 days and @ weaning |
Gilts are homebred in the unit. Boars are used for teasing. Gilts and sows are served with artificial insemination from a single source.
The closest pig unit is 2 km away.
History
The farmer contacted the veterinary surgeon concerning an increase of respiratory distress and loss of body condition with an increase of mortality in the weaning and growing pigs.
Investigation
Clinical investigation
During a farm visit in September 2017, there were many 6 to 15 weeks old pigs with clinical signs of loss of body condition and abdominal panting (Figures 1 & 2). There was very little cough. Pens were overstocked.


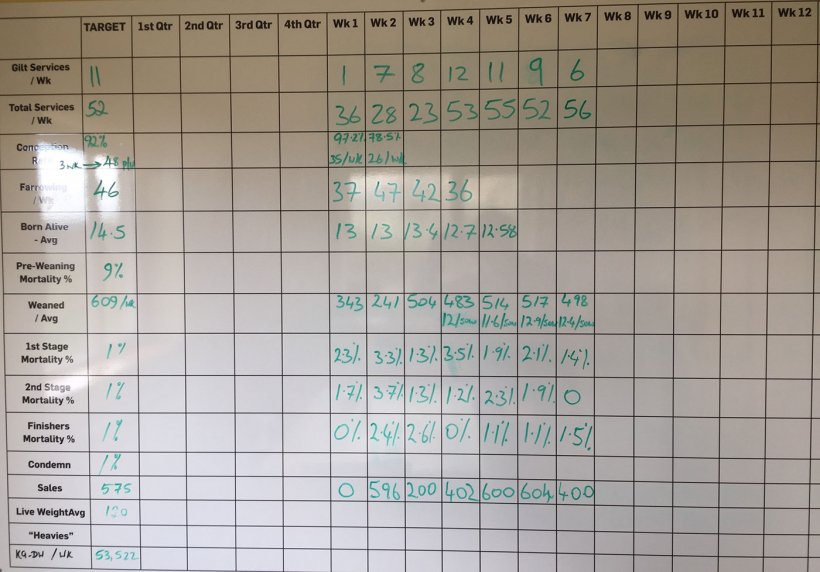
Mortality from weaning to finishing was between 3.5 – 5% when asking to the farmer. However, mortalities were completely different to the ones entered in the weekly wall board of the canteen (Figure 3).
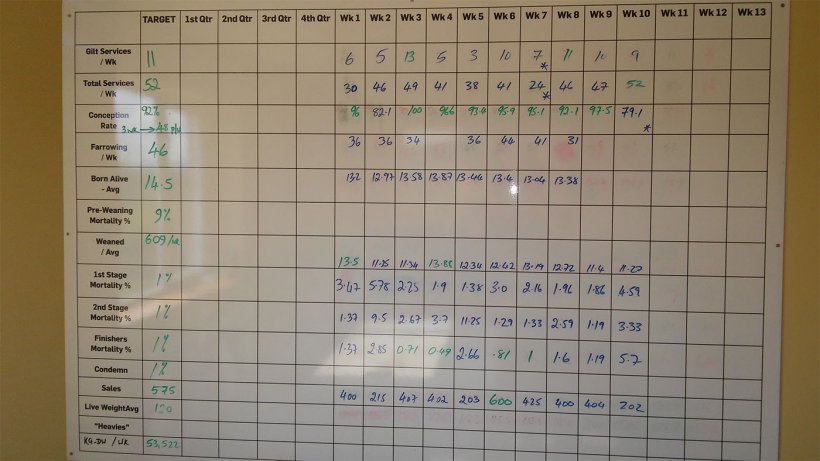
Mortality from weaning to finishing was 8.48% during the last 10 weeks when analysing the data on the board. This mortality was disclosed as 2.64% in 1st stage (from 4 to 9 weeks old); 3.82% in 2nd stage (from 10 to 15 weeks old); and, 1.83% in finishing pigs (from 16 weeks old to slaughter).
Laboratory investigations
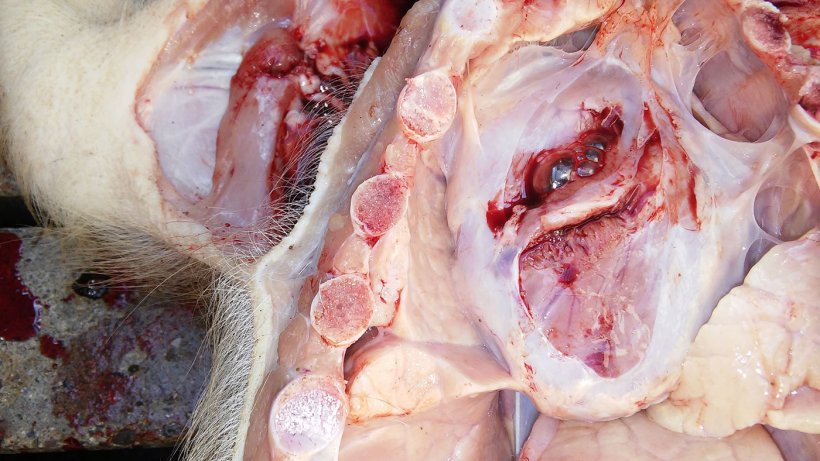
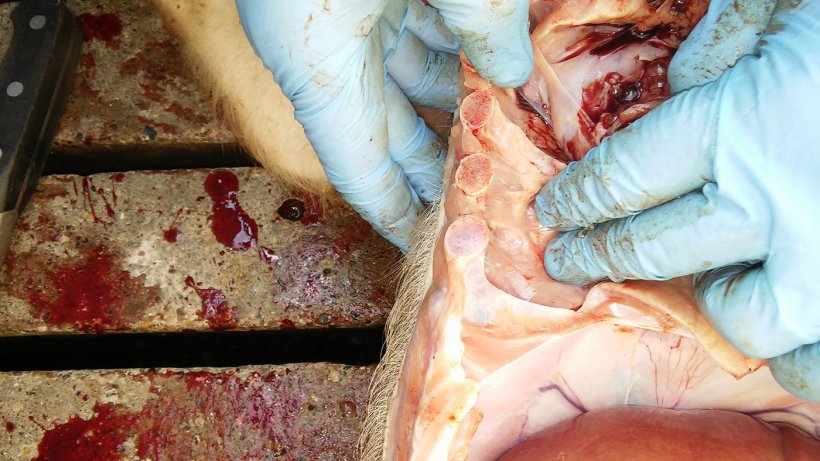
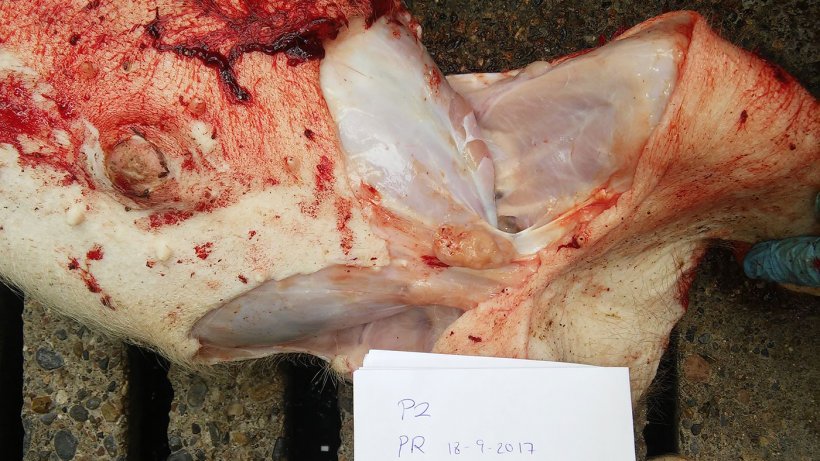
Two 6 weeks old weaner pigs with abdominal panting and loss of body condition were euthanised for post-mortem examination on the farm (Figure 4 & Video 1). Samples were collected and submitted to the laboratory for analyses.

Video 1. Weaner pig with respiratory distress and ill-thrift.
The results of the post-mortem and laboratory investigations are summarized in Table 2. Photographic post-mortem evidence is shown in Figures 5, 6, 7 & 8.
Table 2. Laboratory results from the two 6 weeks old weaner pigs.
| Weaner | Post-mortem | Bacteriological findings | Biomolecular findings | Histology |
| 1 | Poor body condition. Underweight. Pericarditis. Pleurisy. Lung consolidation of the ventral area of the cardiac and diaphragmatic lobes with insterstitial pneumonia. |
Pasteurella multocida. (Lung). Streptococcus suis serotype 3. (Lung). |
Negative to Mycoplasma hyopneumoniae, Influenza Virus type A & PCV-2 by RT-PCR (Lung). EU-PRRS positive by RT-PCR (CT: 21.2) pooled with lung from weaner 1 & 2. |
Bronchi with neutrophilic granulocytes and hyperplasia of the bronchial epithelium. The lung tissue shows different states of a fibrinous bronchopneumonia. Thus, hepatisation and carnification are frequently detected. Proliferation of fibrous tissue is multifocally present in the interlobular and interalveolar spaces. These findings are representative for a chronic inflammation. |
| 2 | Poor body condition. Underweight. Enlargement of the inguinal lymph nodes. Pericarditis. Pleurisy. Lung consolidation of the ventral area of the cardiac and diaphragmatic lobes with insterstitial pneumonia. |
Pasteurella multocida. (Lung). | Negative to Mycoplasma hyopneumoniae, Influenza Virus type A & PCV-2 by RT-PCR (Lung). EU-PRRS positive by RT-PCR (CT: 21.2) pooled with lung from weaner 1 & 2. |
Lung signs of chronic inflammation. Inflammation is more of a catarrhal- purulent type. Bronchi are filled with neutrophilic granulocytes and bronchial epithelium is hyperplastic. Chronicity is characterized by proliferation of fibrous tissue in the interstitial spaces. Pleura are focally hyperplastic and an interstitial oedema can be detected. |

Differential diagnosis
Based on the clinical and laboratory investigations, a list of differential diagnosis was prepared as follows:
- PRRS (Porcine Reproductive and Respiratory Syndrome).
- PCV2 (Porcine Circovirus type 2).
- Influenza Virus type A.
- Enzootic Pneumonia (Mycoplasma hyopneumoniae)
Viral agents, such as Influenza Virus type A and PCV-2, were ruled out on the basis of negative laboratorial results. The vaccination protocol against PCV-2 was satisfactory in this unit.
Enzootic Pneumonia was ruled out as a consequence of negative laboratorial results, satisfactory vaccination program and lack of cough in the feeding herd.
A diagnosis of PRRS infection with secondary opportunistic bacterial infection in the feeding herd was indicated based on the history and consistent clinical and laboratory investigations.
Control programme
Vaccination with a MLV-PRRS vaccine was suggested to all piglets at 10 – 14 days old.
Response to the control programme
There was a reduction of respiratory distress and ill-thrift pigs. Mortality reduced considerable to 5.2% three months later. This mortality was disclosed as 2.25% in 1st stage (from 4 to 9 weeks old); 1.72% in 2nd stage (from 10 to 15 weeks old); and, 1.24% in finishing pigs (from 16 weeks old to slaughter) (Figure 10). Stocking densities were never changed.
Discussion
In the late 1980s, severe reproductive and respiratory disorders were described in the United States (Keffaber 1989). The aetiological agent was unknown at that time. The same syndrome was described in Germany in 1990 (OIE 1992). The aetiological agent was first isolated in The Netherlands in 1991 (Wensvoort and others 1991) and named Porcine Reproductive and Respiratory Syndrome (PRRS) (Terpstra and others 1991).
PRRS infection was first diagnosed in Northern Ireland in 1997 (Anonymous 1997) and in the Republic of Ireland in 1999 (Ohlinger and others 2000).
PRRS is present in most pig producing countries in the World with a few exceptions. Countries such as Sweden (Carlsson and others 2009), Norway (OIE 1997), Finland (Bøtner 2003), Switzerland (Corbellini and others 2006), New Caledonia (OIE 1996), New Zealand (Motha and others 1997), Australia (Garner and others 1997), Argentina (Perfumo and Sanguinetti 2003), Brazil (Ciacci-Zanella and others 2004) and Cuba (Alfonso and Frias Lepoureau 2003) are reported free of PRRS.
Severity of PRRS infection varies from unit to unit. Non- infectious factors exacerbate the expression of clinical signs. These non-infectious factors consist of management, pig flow, housing and temperature regulation (Zimmerman and others 2012). Concurrent infections with some viral and bacterial agents potentiate or add severity of expression of PRRS clinical signs (Shibata and others 2003, Thacker and others 1999, Borobia and others 2014). Pleurisy is one of the lesions caused in the lung of PRRS infected animals (Muirhead and Alexander 1997). BPEX (2009) found the economic impact of pleurisy was substantial. Increasing pleurisy prevalence was associated with reduced carcase weight and increased weight age at slaughter. Cost to the producer, for a typical 10% pleurisy prevalence at batch level were shown to be in order of £2.26 per pig –based on reduced carcase weight and increased age at slaughter.
MLV PRRS vaccination has proven to be useful for limiting the effects of field virus in weaned pigs (Waddell and others 2008), as in this clinical case.








