Today, despite documented efforts to reach acceptable fertility and prolificacy after AI, the cryosurvival of boar spermatozoa is still consistently low, owing to damage during a relatively old processing that is time-consuming (takes about 8-9 h), costly (need cooled centrifuges etc) and yields few doses per ejaculate (often enough for 5-8 sows, 2 AI/estrus). Number of piglets born is yet lower than for cooled or neat semen implying that sperm lifespan, deposition site and closeness to ovulation are conspiring against success. All this does not make frozen boar semen an alternative for pig production, although it is needed for gene banking and export. This note presents alternative freezing processing based on in vivo ejaculate characteristics, where the sperm-peak portion of the boar ejaculate, barely 10 ml is used for freezing of small doses (0.5 ml each), while the rest of the ejaculated spermatozoa (more than 75%) is used for routine production of liquid semen doses, thus combining production and banking. Fertility/prolificacy of these small frozen-thawed doses is acceptable, using deep intrauterine AI during spontaneous oestrus.

Learning from the ejaculate
The boar ejaculate is fractionated, with three distinct fractions visible during manual collection: the pre-sperm fraction (watery, some gel clots, no sperm, about 10-20 ml), the sperm-rich fraction (more dense, containing most spermatozoa, no gel clots, 30-50 ml) and the post-sperm-rich fraction (becoming watery again, ending with gel clots, 150-200 ml). Our own studies have shown that spermatozoa contained in the first 10 ml of the sperm-rich fraction (also called sperm-peak portion, where about 15-20% of all spermatozoa are, Fig 1) were more resilient to handling (from extension to cooling) and cryopreservation that the spermatozoa contained in the rest of the ejaculate. It is considered that the fact that the first spermatozoa bathe in specific secretions (epididymides/prostate) where fertility-associated proteins are present, is behind this increased resilience to handling.
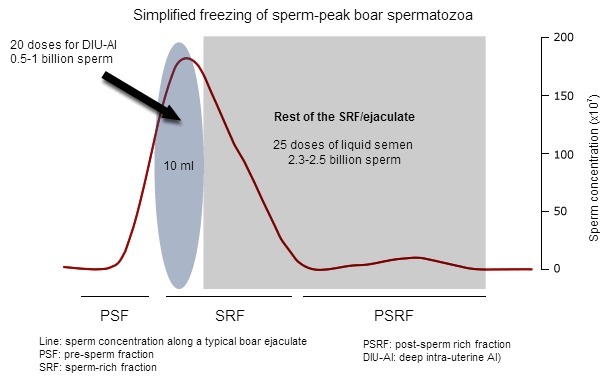
Figure 1: A typical boar ejaculate presents 3 distinct fractions (PSF, SRF and PSRF) depending –among other parameters of the sperm concentration (here as red line). The sperm peak is usually contained in the first 10 ml of the SRF. If frozen, this sperm-peak portion can yield 20 AI-doses for deep.intrauterine AI, while the rest of the ejaculate (often as SRF) can still provide enough sperm for 25 conventional liquid semen AI-doses.
Can we simplify the freezing protocol?
Customary freezing uses the entire sperm-rich fraction, eliminating by extension and centrifugation in cooled centrifuges, the seminal plasma. The sperm pellet is then re-extended, cooled and frozen, often in medium-straws (2.5 ml) or 5 ml-Flat-bags (see Fig 2). AI-doses contain typically 5 billion sperm, thus one ejaculate yielding a maximum of 16 doses for cervical AI (e.g. max 8 sows, 2 AI/estrus). We compared freezing sperm from the sperm-rich fraction (control) with those of the sperm-peak portion (1st 10 ml of the sperm-rich fraction) using a conventional freezing (CF) against a simplified process (SF). Sperm-peak semen was handled in concentrated form (0.9 billion sperm/dose) for eventual use with deep-intrauterine AI (1-2 doses per oestrus, enough for 10 sows). The sperm-peak cells were firstly kept in their seminal plasma for 30 min, and thereafter, without centrifugation (i.e. without removal of the seminal plasma), they were mixed with lactose-egg yolk (LEY) extender and cooled down to +5°C within 1.5 h, before being mixed with LEY+glycerol (3%) and OEP and packed into 0.5 ml Flat-bags´s (see Fig 2). Freezing used in all cases 50°C/min as cooling rate. The entire “simplified” procedure (SF), lasted 3.5 h compared to the control “conventional freezing” (CF), which lasted 8 h; yielding an equally good cryosurvival (above 60% of the processed cells).
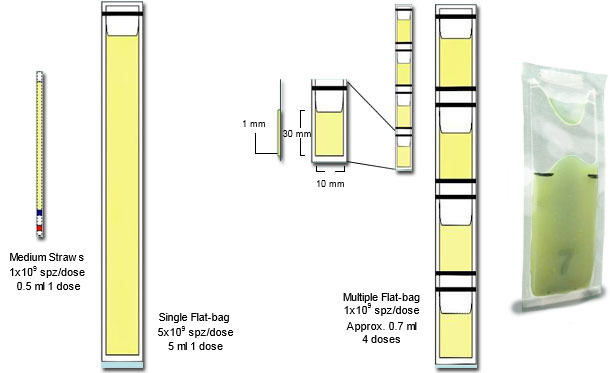
Figure 2: Schematic representation of the major differences between plastic 0.25 ml mini-straws, with single (5 ml) and multiple Flat-bags (0.5-0.7 ml), see adjacent photograph of a filled and sealed mini Flat-bag), courtesy of Dr Fernando Saravia).
What are the advantages?

Several; using this simplified, shorter protocol waives the need of expensive refrigerated centrifuges. Moreover, inter-boar variation is minimised by use of P1-spermatozoa which, not only were the “best” spermatozoa to be cryopreserved, but uses a portion of the sperm-rich fraction where documented “fertility-associated” proteins are present. Finally, the procedure frees the rest of the collected spermatozoa (75-80% of the total sperm count) for additional processing of approx. 25 liquid semen AI-doses (Fig 1). This simpler protocol ought thus to be an interesting alternative for AI-studs to –using the one and the same ejaculate- freeze boar semen for gene banking or for repopulation or commercial distribution, along with production of conventional semen doses for AI with liquid semen, using the rest of the ejaculate. Such procedures would not disturb routine handling of boars or their ejaculates.
But, are these small doses (0.5 ml, 500 million sperm) fertile?
Yes, acceptably fertile, even after single deep-intrauterine AI in spontaneous oestrus. Double deep-intrauterine inseminations (24 and 36 h after detection of standing oestrus) done on 120 sows (2nd parity) with peak-sperm portion semen frozen as above (SF) described, led to 42% farrowing of 8 piglets alive per litter. Complementary doses of liquid semen (2.3 x 109 sperm) led to 80% farrowing and 13 piglets alive/litter (M Wallgren, unpublished results). Although not an alternative to displace liquid semen for commercial use, this freezing procedure is a good alternative for gene banking and biosecurity checks without disrupting commercial production of AI-liquid doses or intervention on boar handling.







