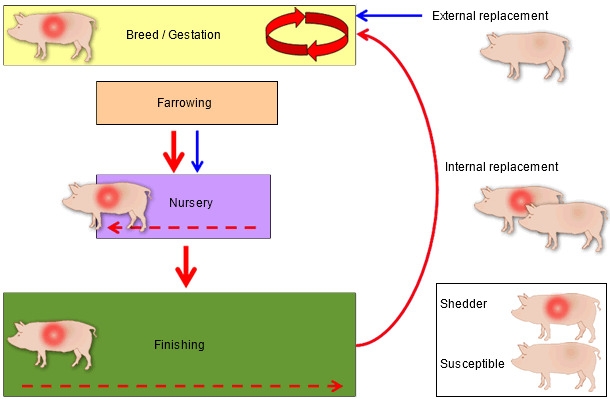
After the introduction of porcine reproductive and respiratory syndrome virus (PRRSV) into a breeding herd, reproductive failure and increase in mortality are usually observed (Loula, 1991). Within a few months, the production parameters may return to levels similar to those prior to the infection; however, the co-existence of persistently infected and susceptible subpopulations perpetuates viral transmission within herds (Dee et al., 1996). The virus can replicate in both negative and previously infected gilts if they are mixed with shedding individuals in the breeding herd (Figure 1). This dynamic causes the continuous weaning of infected piglets that usually become clinically affected following comingling and maternal immunity decay. The actively infected nursery pigs represent a risk of infection for the source sow population and for neighboring farms. Economic studies indicate that it is in these post-weaning pigs and not in the sows where the majority of the cost associated with PRRS is generated (Neumann et al., 2005; Holtkamp et al., 2011).
Figure 1. Mechanism of perpetuation of PRRSV infection in a breeding herd

In countries where consensus about the economic impact of the disease has been reached, the standard goal for controlling PRRS is to consistently produce negative weaned pigs. The American Association of Swine Veterinarians activated a committee leaded by Drs Holtkamp and Polson in 2011 to develop a standard terminology to classify herds according to PRRSV infection status. The classification describes testing recommendations to define herds from Positive (category I) to Negative (category IV) during the stabilization or elimination process. A herd is classified as Stable (category II) when serum samples from at least 30 due to be weaned piglets pooled in groups of five samples test PCR-negative in four consecutive monthly samplings (120 serum samples = 24 PCR tests equally distributed through 90 days). Once the herd is stable ELISA negative gilts can be mixed with the previously infected population with relatively low risk of infection. If 60 gilts test negative for individual ELISA test two months after they have been introduced to the population, the herd can be now defined as Provisionally Negative (category III). When all originally infected (ELISA positive) sows have been replaced, “rolled-over”, for ELISA negative gilts with no evidence of virus infection the herd can be classified as Negative (category IV).
Depopulation of infected pigs and repopulation with negative pigs, as well as, test and removal have been proposed as methods to eliminate PRRSV from breeding herds; however, the most cost-effective procedure to eliminate the virus from infected populations is herd closure or load-close-homogenize (Yeske, 2011). The protocol is applied to acutely or endemically infected breeding herds and usually begins with the introduction of as many gilts as the facilities can house (LOAD), followed by the immediate interruption of internal or external gilt or boar replacement until the time that the herd is defined as stable (CLOSE). Finally, the simultaneous exposure of every adult pig in the population to PRRS live resident virus or modified-live virus vaccine is performed once or twice (HOMOGENIZE). A variation of the procedure, called “off-site breeding”, consists on having a separated facility where negative gilts are raised and bred to be moved to the breeding herd only when stability has been confirmed, or the gilts can be infected with the same virus isolate used to homogenize the breeding herd exactly at the same time. Biosecurity and monitoring protocols are critical to avoid introduction of PRRSV with these gilts.
Figure 2. Example of procedures and wean-pig PRRSV detection during herd closure
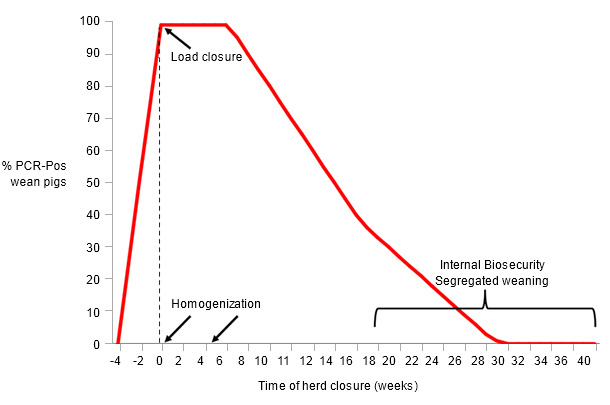
The proportion of PCR-positive pigs at weaning begins to decay a few weeks after the homogenization of the herd is performed (Figure 2). At some point during the closure, most likely after 16 weeks, the pigs are born non-viremic but infection occurs during lactation at a very low rate (Cano et al., 2008). Therefore, strict internal biosecurity to minimize transmission in the farrowing rooms and effective weaned pig segregation is critical to complete the elimination of PRRSV from the herd. A recent study from the University of Minnesota revealed that herds take 30 weeks (12 to 43 weeks) to consistently wean PCR-negative pigs after herd closure was initiated. The authors found that the method of virus exposure and the previous infection status significantly affected the time needed to achieve stability (Linhares et al., 2012). It is important to differentiate herd closure and closed herd. A closed herd does not introduce new pigs from other farms but still “recycles” internal weaned pigs for future replacement. That farrow-to-finish and continuous flow operation usually has a lower risk of introduction of new infectious agents from the outside but has a higher risk of recirculation of those microorganisms already present.
With slightly different protocols, veterinarians in North America feel confident about the current methods to eliminate PRRSV from a herd; however, the difficult question typically resides on what to do with the new gilts once the virus has been eliminated.
“Should the gilts be negative, vaccinated or infected with resident virus?”.
The decision about the replacement gilt acclimation program for PRRS can be oversimplified based on the risk of a new infection in the breeding herd. If this risk is assumed to be a function of the local pig density, the surrounding PRRSV prevalence and the biosecurity level of the farm, three situations and potential interventions can be proposed:
| Risk | Low | Medium | High |
| Local pig-density & PRRS-prevalence | Very low | Low-medium | Medium-high |
| Biosecurity infrastructure & protocols | Excellent | Acceptable | Poor |
| Expected frequency of PRRS outbreaks | Rare | Every 2 or 3 years | Every year or more |
| Company PRRS infection status | Negative | Negative-Stable | Stable-Positive |
| Production goal | External multiplier | Internal multiplier or commercial | Commercial |
| Action plan following a PRRS outbreak | Elimination | Stabilization | Control |
| PRRS diagnostics in weaned pigs | PCR/ELISA-negative | PCR-negative | PCR-negative/positive |
| PRRS status of external gilts/semen | Negative | Negative | Negative |
|
Gilt isolation period (wks) |
6 |
3 |
2 |
| Gilt immunization (PRRS acclimation) | No | Modified-live and/or live-resident virus | Modified-live virus |
| Gilt recovery (“cooling”) period (wks) | No | 14 | No |
Farms located in areas with low pig density and/or prevalence of infection and with adequate biosecurity to prevent diseases, PRRSV elimination is indicated. When disease introduction has been rare and the goal is to produce negative pigs, replacements can stay negative and monitored by a comprehensive surveillance program. In the other hand, because the consequences of a PRRS outbreak in negative herds are usually significantly higher than in previously exposed herds, the immunization of the replacement gilts in herds with moderated to high risk of infection is greatly recommended. Despite the knowledge gap regarding complete heterologous protection in breeding herds and the difficulty to predict the next PRRSV isolate infecting a population, recent studies show that previous immunity not only reduces the time needed to achieve stability but also the time to return to the production level before the infection and minimizes the production losses during the outbreak (Linhares et al., 2012).
Although exposing gilts to old sows, viremic piglets or tissues has been proposed as an acclimation method, the difficulty to determine the dose and time of infection seriously limits the use of such strategy. The injection of gilts with wild-type live virus has been commonly used for exposure during acclimation. Also, the use of modified-live virus vaccine to immunize gilts during acclimation has been widely implemented. The severity of clinical signs and the ability of the process to effectively expose but avoid introduction of shedding gilts to the breeding herd need to be considered to decide between modified-live or wild-type virus. The recovery phase of the acclimation period in a stable herd needs to be of at least 100 days in all-in / all-out groups to avoid virus recirculation. When the risk of infection is high the feasibility of performing extended herd closure every time a new PRRSV enters the herd is compromised. In those cases maximizing immunity and minimizing exposure through a systematic use of vaccination in gilts at arrival and one month later and vaccination of piglets at weaning might be the most beneficial decision.
The desired result of the strategic management of replacement gilts at a company level is to minimize the economical losses potentially caused by PRRS. An integrated measurement of success is to calculate the average number of weeks weaning PCR-negative pigs per breeding herd in the entire system. The negative pigs have a better chance to perform than positive pigs, even if they need to be placed in high pig density and PRRS prevalence areas, because the vaccination at weaning will allow developing active immunity usually before any lateral challenge.