Streptococcus suis (S. suis) is considered one of the most important re-emerging swine bacterial agent in the era of antimicrobial restrictions. Indeed, S. suis infections are one of the main causes of antimicrobial usage in piglets, either curative or (where allowed) prophylactic/methaphylactic.

Worldwide data of S. suis antimicrobial resistance are worrisome; therefore, to reduce antimicrobial use, disease prevention should concentrate on management of predisposing factors and vaccination. Despite intensive research leading to different vaccine-candidate antigens, no universally efficacious S. suis vaccine has been commercialised so far. More research would certainly advance sub-unit vaccine development. Meantime, the only available vaccines used in the field are autogenous, which consist of killed bacteria (“bacterin”) from the predominant isolate(s) recovered in an affected farm/system, produced by licenced laboratories and given back to the same farm/system. However, there are very few scientific studies demonstrating whether the use of such vaccines in the field correlates with a reduced mortality and curative antimicrobial use. Indeed, field peer-reviewed reports on autogenous vaccines are almost non-existent - only 4 published papers in the last 30 years. In addition, controlled laboratory studies have shown contradictory results concerning protection induced by experimentally-produced bacterins (for a review see Rieckmann et al. 2020).
The unresolved issues on S. suis autogenous vaccines
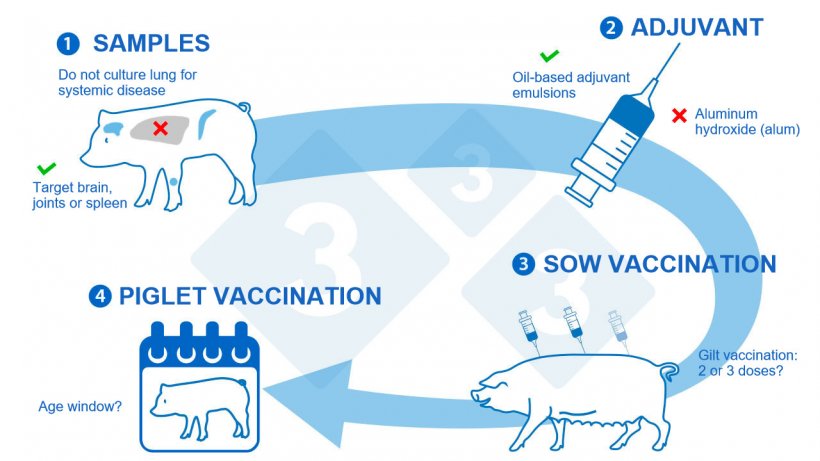
1. S. suis diagnosis – at the beginning and at the end: The correct diagnosis of S. suis as a primary cause of disease may complicate the choice of the isolate(s) to be included in the autogenous vaccine. There is still an unresolved issue concerning those recovered from lungs (Obradovic et al, 2021), which are considered as secondary invaders and should probably not be included in the vaccine composition. Proper S. suis diagnosis is also important to evaluate the effectiveness of the autogenous vaccination program applied to the farm. An important confounding factor is the assessment of total mortality or total treatments instead of those directly related to clinical signs compatible with S. suis disease. It should be considered that other bacterial infections may induce similar clinical signs, such as Glaesserella (Haemophilus) parasuis. In addition, if an outbreak of an unrelated disease was present on the farm and further controlled during the vaccination trial, the improvement in health might not be directly linked to the vaccine effect. The opposite, such as a concomitant infection causing piglet mortality, might also negatively affect the evaluation of the autogenous vaccine. Therefore, confirmatory necropsy, followed by bacteriology and S. suis serotyping should be systematically performed in vaccine field studies.
2. Vaccine formulation – the secret to success: Autogenous vaccines are “manufacturer-related” as each licenced laboratory uses different protocols, antigen doses, types and concentrations of adjuvants, among other variables introduced during the manufacturing process. Adjuvants are key components of a vaccine: they have the power to modulate the vaccine efficacy, the strength and duration of the vaccine-induced immune response. Despite the importance of adjuvants, few studies have compared their effect within the same experimental trial or at least under the same conditions. A recent work comparing the effect of six different commercial adjuvants on S. suis bacterin vaccine efficacy, showed that the type of adjuvant has a paramount effect on the immune response and protection of piglets against a S. suis challenge. This study also confirmed previous findings on the limited immunogenicity and/or protection of bacterin vaccines formulated with aluminum hydroxide, commonly known as alum; while the use of oil-based adjuvant emulsions as adjuvants shows promise. Therefore, more studies are necessary on the effect of vaccine formulation.
3. Sow vaccination – the dilemma of numbers of doses. Immunization of sows might elicit protective passive maternal immunity in the progeny. Sow vaccination is less costly and labor intensive, thus representing an economical alternative to piglet vaccination. A recent field study reported that a 3-dose program in replacement gilts was required to reach a significant increase in antibody levels (Corsaut at al, 2021); providing for the first time a scientific justification for implementing such a program in external replacement gilts entering quarantine. This vaccination program resulted into a higher maternal immunity present in piglets when compared to those from non-vaccinated gilts. In another field study (Corsaut at al, 2020), sows from internal replacement, which have received a 2-dose autogenous vaccine program also showed increased levels of antibodies. However, maternal antibody transfer to piglets and thus their clinical protection at the nursery was not improved. These discrepancies might be explained by several variables, including the vaccine formulation, the use of 3 doses vs. 2 doses, and internal vs. external replacement sources among other herd-specific factors. In spite of these differences, a common feature observed between the two field studies was that duration of maternal immunity drops very fast independently of the vaccination program. This drop in maternal immunity occurs at the moment of high vulnerability of weaned piglets to S. suis infection. Therefore, the question remains on how improving the duration of maternal immunity to protect piglets during the complete nursery period.
What about a “boost dose”? This is another common practice in the field but no scientific data are available to support this prevention strategy. In one of the aforementioned studies (Corsaut at al, 2021), a “boost” vaccine dose to the originally vaccinated gilts resulted in a recall (“memory”) response in terms of antibody levels at subsequent parities. Yet, the protective effect on the progeny remains to be elucidated. Similarly, the effect of a combination of mass vaccination with repeated doses of autogenous vaccines before each parturition has not been yet scientifically studied.
4. Piglet vaccination – too young or too old: To the best of our knowledge, only three published articles have addressed the efficacy of this preventive approach in the field. In a farrow-to-finish farm, piglets received an autogenous vaccine at weaning and a booster 3 weeks after entering the nursery. The direct effect of vaccination (mortality due to S. suis) was not statistically significant. However, the calculated total and overall vaccine effectiveness (complete herd-level mortality) showed somehow potential protective effects; yet mortality due to any cause was considered here (Hopkins et al, 2019). In a field study where piglets were vaccinated at weaning and boosted 10 days later, mortality and morbidity rates in nursery pigs fluctuate regardless of treatment (Torremorell et al, 1997); confirming the difficulty in assessing the clinical outcome of vaccination and the importance of proper diagnosis. Finally, in the third study, piglets received an autogenous vaccine during the first week and at three weeks of age. This vaccination program failed to induce an antibody response and no clinical protection was observed. The lack of response may be due to interference with a high level of maternal antibodies and/or an immature immune system of very young piglets. Therefore, more research is needed to evaluate the perfect age window for piglet vaccination in order to avoid maternal interference but confer protection at the moment of S. suis clinical signs onset.
Conclusion
In spite of decades of research on S. suis vaccines, autogenous bacterins are almost the only preventive strategy available that swine producers have access to. Therefore, more field studies are essential to scientifically validate their protective effect and, consequently, their cost-benefit impact for swine producers. In addition, more experimental (laboratory) studies are required to generate scientific knowledge to improve this important preventive tool and help reducing the use of antimicrobials.