There are several decisions to make before attempting an eradication programme. Firstly, which antibiotic should we use? If we have had the disease on the farm for some time, the Brachyspira will have been exposed to possibly a number of drugs and may have reduced susceptibility to some of them. It is a very good idea to isolate the organism by culturing infected faeces and then carrying out a minimum inhibitory concentration (MIC) test on the various antibiotics that you plan to use. If the test is using agar plates containing different concentrations of the antibiotic, this tends to give you a higher MIC reading but some authorities have described this as being equivalent to the MBC (or minimum bactericidal concentration) as no organisms survive on the plate. This is important as we need to use sufficient antibiotic to kill and eliminate the bacteria. Another test uses broth and this commonly gives a true MIC, which is frequently half the level of the MBC. If a sufficient number of isolates are tested, they tend to fall into a susceptibility pattern, which is often moulded by antibiotic use and show the mutations of the bacteria, which may lead to complete resistance. These patterns were described by Karlsson et al (2002) using 76 Australian field isolates.
We also need to know what colon contents concentrations (CCC) the antibiotic achieves in the large intestine and at what inclusion level in the feed or dose rate administered in mg/kg bodyweight are required (see Figures 1-5).
Figure 1. Concentrations of tiamulin achieved in the large intestine (CCC) at different inclusion levels against the susceptibility pattern of B. hyodysenteriae(from Karlsson et al, 2002)
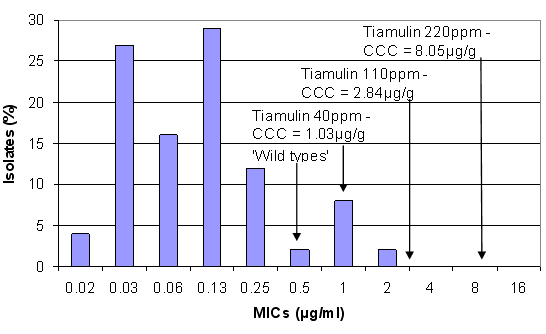
Tiamulin has been most commonly used for swine dysentery eradication programmes and has a good record of success. From the susceptibility pattern the ‘wild type’ strains are at about 0.5µg/ml. This refers to the normal pattern of isolates, which have not been previously exposed to antibiotics. As the concentration of drug is increased in the feed a larger quantity is taken in by the pig and a larger quantity accumulates in the large intestine, where the Brachyspira live. Most of the drugs that are used to treat Brachyspira are termed bacteriostatic, i.e. they inhibit the growth of the bacteria but do not necessarily kill or eliminate them, unless higher concentrations are used. To achieve bacterial kill, which is required for eradication, concentrations in the gut at 8-10 times the MIC or 4-5 times the MBC are required over a sufficiently long period – usually 2 weeks – to completely eliminate the bacteria. Using tiamulin as an example, 40ppm tiamulin achieves a CCC of 1.03µg/ml (which is equivalent to 2mg/kg bodyweight) would inhibit the growth of the wild type bacteria but would not necessarily kill them all except for the most sensitive say 0.13µg/ml or below. Tiamulin at 220ppm in the feed, which achieves 8.05µg/ml however will kill organisms with MICs of 0.5-1.0µg/ml. High doses/inclusion rates are required to kill the bacteria, so these levels frequently give the best results.
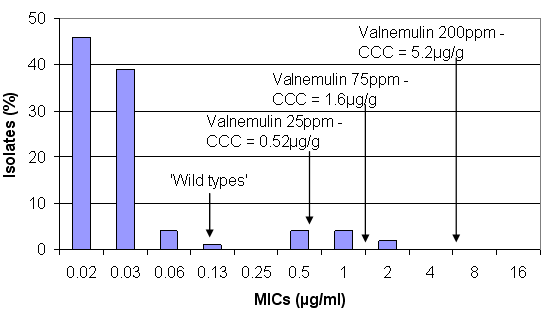
Valnemulin is another pleuromutilin antibiotic, which is also highly active and effective against B. hyodysenteriae (see Figure 2). The prevention level will normally inhibit the wild types but higher concentrations of 75ppm are required to treat the wild types and the pneumonia treatment level of 200ppm would also treat some of the first step mutants and inhibit most of them.
Figure 2. Concentrations of valnemulin achieved in the large intestine (CCC) at different inclusion levels against the susceptibility pattern of B. hyodysenteriae (from Karlsson et al, 2002)
Lincomycin has also been commonly used in the past, but resistant isolates have been more frequently described (see Figure 3).
Figure 3. Concentrations of lincomycin achieved in the large intestine (CCC) at different inclusion levels against the susceptibility pattern of B. hyodysenteriae (from Karlsson et al, 2002)
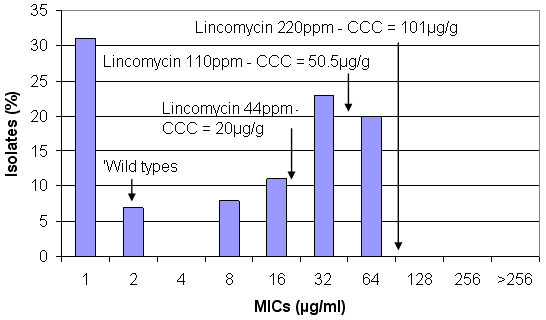
It has a slightly different pattern from the pleuromutilins, with the wild types being at around 2µg/ml, the prevention dose of 44 ppm is thought to achieve concentrations of about 20µg/ml, which inhibits the wild types and some of the 1st-step mutants but very high concentrations – the respiratory treatment level of 220ppm would be required to inhibit all of the mutants but is unlikely to eliminate a large number of them.
Tylosin, a macrolide, has developed a major resistance pattern (see Figure 4) and is rarely used for eradication programmes.
Figure 4. Concentrations of tylosin achieved in the large intestine (CCC) at different inclusion levels against the susceptibility pattern of B. hyodysenteriae(from Karlsson et al, 2002)
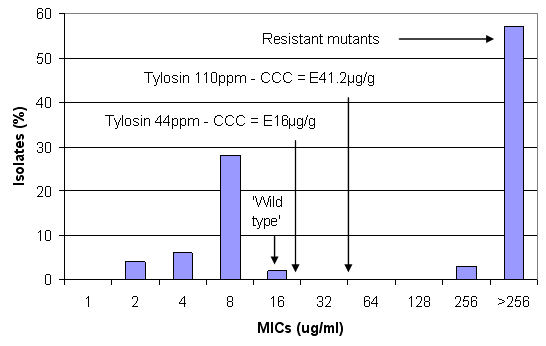
Tylosin at 44ppm is thought to achieve sufficient concentrations to achieve inhibition of the wild type strains but it is considered unlikely to treat them. At 110ppm tylosin, concentrations in the large intestine can be estimated at about 41µg/ml and therefore might eliminate very sensitive isolates with MICs of 4µg/ml.
A newer macrolide, tylvalosin, has a different susceptibility pattern to tylosin but one that is more similar to lincomycin. Unfortunately, the data is not directly comparable as the data on 31 Swedish isolates was from a different study (Karlsson et al, 2004) (see Figure 5). There were fewer isolates than in the previous study and some resistant isolates were included on purpose. The work was carried out using the broth method also, which was similar to the earlier study and it gives a good indication of susceptibility.
Figure 5. Concentrations of tylvalosin achieved in the large intestine (CCC) at different inclusion levels against the susceptibility pattern of B. hyodysenteriae (from Karlsson et al, 2004)
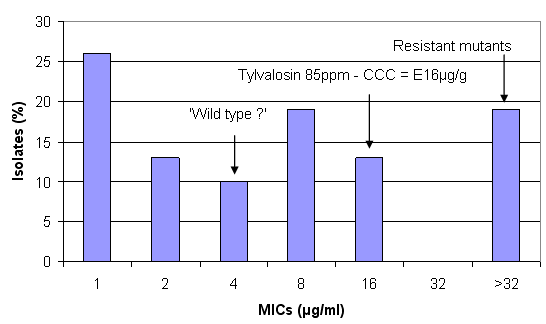
Tylvalosin at 85ppm in feed accumulates in the CCC at a concentration that can be estimated at approximately 16µg/ml. Isolates with an MIC of 16µg/ml and below, therefore, should be inhibited by tylvalosin but possibly only the very sensitive isolates would be eliminated.
The most successful programmes for eradication have been based on partial depopulation of all of the growing pigs and focusing the medication on the sows and possibly suckling pigs. Eliminating it from grower and finishing pigs is usually much more difficult and expensive and it also gives a good opportunity to clean and disinfect the buildings and carry out repairs, especially to the flooring.
Planning is absolutely critical. Clean up the farm as much as possible, get rid of dung heaps, clutter and other rubbish lying around the farm, which can harbour vermin especially rats and mice. A rodent and fly control programme should be introduced. Biosecurity protocols should be introduced.
The 8-week medication was originally divided into a 2-week high level drug to eliminate the infection, a 2-week intermediate level to treat any further cases but to prevent any re-infection should there be any contamination, followed by a 4-week prevention period (see Figure 6), to stop any break back of infection from the environment.
Figure 6. Various sow medication programmes to eliminate swine dysentery
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Original programme |
High | HigH | Medium | Medium | Low | Low | Low | Low |
| Adapted programme | High | High | High | High | Medium | Medium | Medium | Medium |
Tiamulin dose in feed: High = 6-10mg/kg; Medium = 5mg/kg; Low = 2-3mg/kg bodyweight
Dry sow feed intakes are low (1% of bodyweight) and lactating sows intake is about 2.5% of bodyweight, so inclusion levels need to be increased, as they are based on growing pigs eating 5% of bodyweight (ie 200ppm in feed gives 10mg/kg bodyweight).
Last year we had a number of herds in the UK break down with swine dysentery. One unit could not properly remove the growing pigs, so they remained on site; a second herd broke in October and was also an outdoor herd, so the site could not be cleaned. In these cases an adapted programme was introduced, where the high medication was used for 4 weeks and the medium medication level was continued for a further 4 weeks because the risk of incomplete elimination of Brachyspira from the environment and possible reinfection was much higher.
There are a variety of ways to eliminate swine dysentery from a herd but it is becoming increasingly difficult to achieve, as there are a limited number of antibiotics to do the job. Hopefully, producers will actively consider taking the opportunity this summer, whilst there are still antibiotics available to which B. hyodysenteriae is still susceptible.




