Swine Influenza used to be known as a sporadic infection affecting swine farms once or up to three times a year in densely populated areas. However recent studies have shown that swine Influenza viruses of type A (IAV-S) can settled within farrow-to-finish farms and persist for a long time with recurrent infections in every batch at fixed age.
A study has been carried out by epidemiologists and virologists from Anses Ploufragan laboratory, in France, in three persistently infected farrow-to-finish farms which were vaccinating the sow herd against IAV-S but were still affected by those recurrent influenza infections at fixed age. The vaccination scheme included a primo-vaccination of gilts during acclimatization (2 injections) with a systematic booster injection in sows 2 weeks before farrowing. The study consisted in a detailed follow-up of three consecutive batches from piglets’ birth until slaughter of the monitored growing pigs. During this follow-up, samples were taken from a random sample of piglets to detect IAV-S (daily nasal swabs during influenza-like outbreaks) and other infectious agents. Characteristics of monitored piglets (quality of maternal immunity, assessment of the post-infectious immune response, husbandry practices) have been analyzed to further understand factors associated with recurrent IAV-S infections in farrow-to-finish farms.

Epidemiological characteristics of recurrent influenza infections
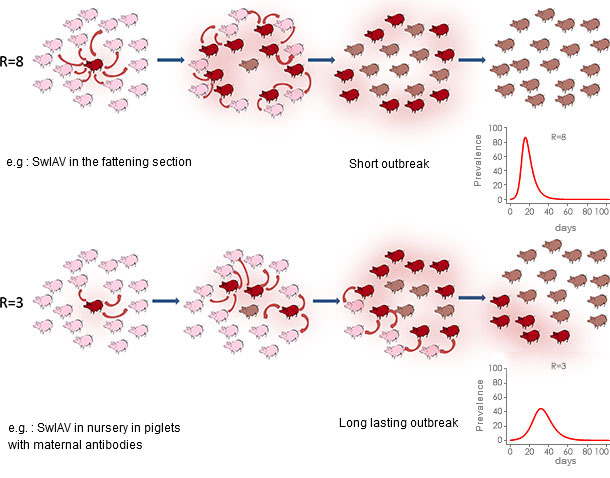
The results showed that this epidemiological form of influenza infection occurred more frequently in the nursery than in the fattening section even if one of the three monitored herd was affected twice in every batch (nursery and beginning of fattening period). In the three studied farms, one or several influenza-like outbreaks were observed in each batch with an remarkable repeatability as regard the average age at infection time. In those farms, simultaneous or delayed co-circulation of different IAV-S subtypes were observed involving the main subtypes circulating in the area (Brittany): H1avN1 (avian-like lineage) and H1huN2 (human-like lineage). Those viruses spread rapidly within the population (high reproduction ratio [R0], see figure 1). Both sub-types can be responsible for the emergence of reassortant viruses when co-infecting a pig at the same time, and it was observed in one batch of Farm#3. A previous infection by one subtype did not confer cross-immunity towards another subtype which led to consecutive infection of the same piglets by those two subtypes with associated clinical symptoms.
During influenza outbreaks detected in this study, the first shedding piglets were mainly those which did not receive high levels of maternal antibodies specific to IAV-S. Those piglets were mainly coming from litters with a proportion of cross-fostered piglets and were mainly born to first parity sows. However passive immunity although tending to delay virus shedding, did not fully prevent from infection neither virus excretion nor IAV-S transmission between penmates. The transmission of the virus was reduced but not enough to prevent from propagation within the population. Moreover, compared to infections occurring in fully susceptible animals, the infection spread more slowly leading to a global infectious process extended at the population level (Figure 1). In addition interference was found between the presence of maternally derived antibodies (MDA) and the post-infectious humoral immune response, resulting in the absence of production of detectable antibodies in those piglets infected in presence of MDA. However, although no antibodies were produced by these piglets, it couldn’t be demonstrated that they remained fully susceptible to the same virus. Those piglets infected in presence of maternal antibodies also tended to shed the virus for a longer time. Co-infections (Mycoplasma hyopneumoniae, PRRSv, PCV2) were not found being at the origin of the recurrent process even if clinical consequences were extremely enhanced in case of dual PRRSv + IAV-S infection.
Reproduction ratio (R0) in epidemic or endemic context
Tools for IAV-S reccurent infections control
According to the complexity of the infectious process involving several viruses and the interference of maternal immunity with the post-infectious immune response, control strategies rely mainly on good husbandry practices and reinforcement of internal biosecurity. These infectious outbreaks can last for more than a month at a population level, which explain that a new batch including mainly susceptible piglets can get infected if the animals are housed in a separate room but in the same compartment (airborne transmission). Strict segregation of batches is therefore of paramount importance with verification that airborne transmission is not possible from one room to another according to the conception of the ventilation system.
Mingling of animals of different immune and infectious status should be therefore prohibited at the different key stages (cross-fostering in farrowing sector, mingling at weaning, mingling during the nursery period). Air communication and movements of people between sectors must also be thought in a consistent way to prevent contacts between young animals and older ones.
IAV-S circulate in the breeding herd of these farms even in vaccinated animals and it is very difficult to identify because duration of shedding is reduced and symptoms are generally absent. In order to prevent from regular re-exposure of sows and to progressively prevent from persistent circulation of the IAV-S in this sector, it is important to separate physically sows from growing pigs within the farm. This separation includes the animals themselves (circulation between these two sectors), farm staff (overalls, specific boots), equipment, and air communications.
Strict application of these control measures can improve the situation in farms with batch rearing systems allowing quiet large intervals between two batches. In herds reared on a weekly-based or 2 week-based intervals between two batches, it may be necessary to implement a breakdown of the cycle through the export of one or two batches of piglets at weaning in external facilities if the possibility exists. Farms located in densely populated pig areas are also exposed to an important risk of contamination from outside due to airborne transmission from neighbor herds or from trucks transporting swine. External biosecurity is therefore pivotal as regards the risk represented by pig transportation as well as rendering vehicles.







