Who hasn’t blocked all the drinkers of a nursery after having established a water medication treatment? How many times have we blamed the product used for the treatment?

Blocking the drinkers isn’t the worst thing that could happen, actually, if it does happen then we know the pigs haven’t had any water, or medicine. But sometimes water medication treatments fail seemingly without reason. In these situations doubts arise: we begin to doubt the product, the dosage, the employee who applied the treatment or even the diagnostic, upon seeing negative results.
In order for a molecule to be water soluble it should be capable of self ionization; if it doesn’t possess radicals capable of ionization it will precipitate and settle on the bottom if treating a tank. This is what will happen if we try to use a ‘premix’ in the drinking water.


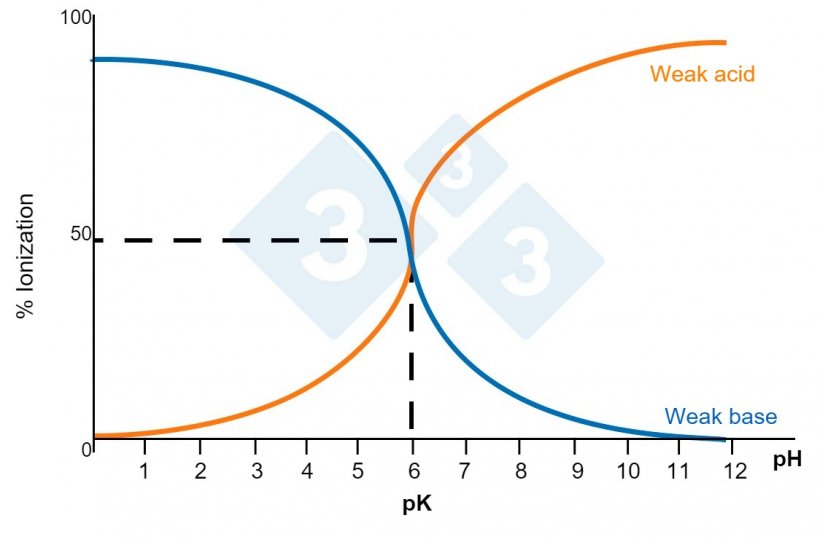
A molecule capable of self ionization when coming into contact with water would be, for example, a salt, and this is one of the most common presentations of soluble medications. A salt will separate itself into two types of radicals: acid (positive) and basic (negative). Not all molecules used will separate into the same quantity of acid and basic radicals. The characteristic of separating into more or less acid radicals is expressed through the constant pKa. The smaller this constant is, the more acidic the molecular character will be. So, with a pKa of 2,7 (that of phenoximetilpenicillin) the molecule will be considered acid, while with a pKa of 7,6 (that of lincomycin) it will be considered basic. When the pH of the medium in which it is dissolved coincides with its pKa, the molecule will become 50% ionized. In order to reach a good solution, the molecule should be fully ionized. So,
- a molecule that possesses a weak basic character will better ionize in an acidic pH (granitic water)
- a molecule with a weak acid character will better ionize in a basic medium (calcareous waters).
Among the molecules that we can classify as weak acids we can find: ampicillin, fenoximetilpenicilina, amoxicillin, quinolones, etc.
Among the molecules that we can classify as weak bases we can find: macrolides, lincosamides, tiamulin, tetracycline, etc.
In practice, slightly acidifying or neutralizing drinking water can be interesting when trying to improve the solubility of the products used.
Tip: In order to avoid problems with weak base molecules such as tetracycline, acidifying the drinking water would be a recommended measure.
In the case of substances classified as weak acids, such as amoxicillin, ampicillin or phenoximeltilpenicillin, avoiding their use in acidified water (common in early phases like post-weaning) is recommended.
Actually, strongly acidified waters (pH < 5) could even limit the efficacy of these substances, affecting any possible results obtained from these medications.













