The integrity of epithelium and the mucous secretion represent the first physical line of defense against antigens, toxins and microorganism. Furthermore, epithelial and innate immune cells secrete antimicrobial proteins (AMPs) in response to pathogens.
The most important defense against opportunistic or primary pathogens is represented by the innate and acquired immune cells associated to mucosal surface, which are organized either in well-defined structures called Mucosal-associated Lymphoid tissues (MALT), e.g. Gut-associated Lymphoid Tissue (GALT), PP (Peyer’s Patches), BALT (Bronchus-asscoiated Lymphoid Tissue), NALT (Nasopharyngeal-asscoiated Lymphoid Tissue), etc.), or scattered in the epithelium and in lamina propria.

Expression of PRRs, mainly TLRs and NOD-like Receptors (NLRs), on epithelial and innate immune cells modulates the recognition and inflammatory/immune response against microorganisms.
Intestinal mucosal immunity develops from birth, in relation to microbiota colonization that drives immune maturation and expansion of GALT structures, influencing the occurrence of effector lymphocyte subpopulations and B cells involved in IgA production. Different subpopulations of CD4, CD8 and γδT lymphocytes and innate immune cells localize in the lamina propria and the epithelium (Figure 1).
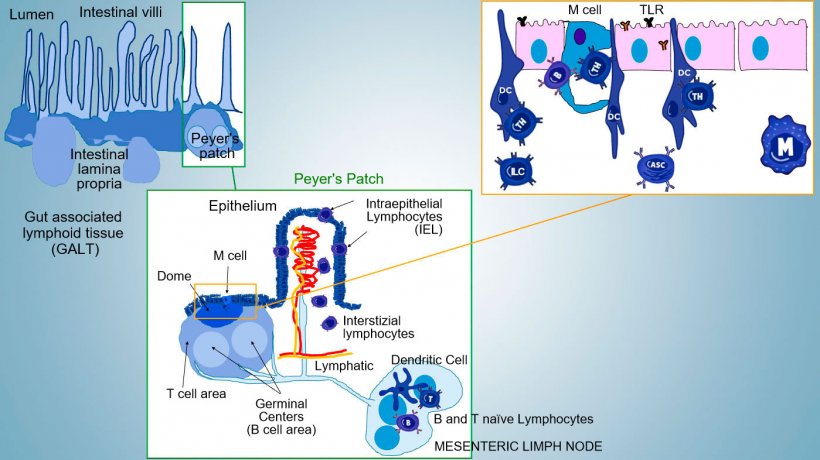
The most important Thelper subsets differentiating in the intestinal propria are regulatory T cells (Treg), Th1, Th2, and Th17 lymphocytes. Innate Lymphocytes Cells (ILCs) also play a key role in immune responses at barrier surfaces, interacting with other innate and acquired immune cells, mainly with Th17.
In addition, intestinal microbiota, inhibits colonization and overgrowth of pathogen by providing signals that substain some innate mechanism, e.g., antimicrobial proteins secretion.
In lung, CD4 Th1 and CD8 cytotoxic are present as effector cells against intracellular pathogen but also other subsets including Th2, Th17, Treg, and TFh are involved.
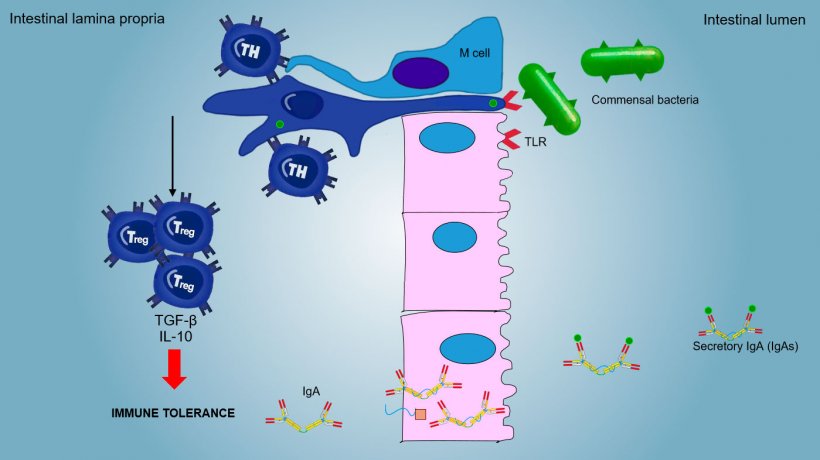
The mucosal immunity is able to discriminate between commensal microorganisms, food or aerosol antigens and primary pathogens.
Aerosol and food antigens and commensal flora generally do not stimulate an inflammatory/immune response but they induce an immune tolerance (Figure 2).
Functionally, the immune responses at mucosal surfaces are distinguished in inductive and effector sites, in relation to:
- Inductive sites: PP, isolated lymphoid follicles (ILF), NALT, BALT, mesenteric lymph nodes (MLN). Sample antigens and activate immune response.
- Effector sites: mucosal lamina propria of gut, upper respiratory tract, genito-urinary tract, mammary and salivary gland. Generate a defensive response inducing both inflammatory/innate and specific immune responses.
In inductive sites, antigens are captured by microfold cells (M) which transport internally and release antigens into the extracellular space, where they are captured and processed by the underlying dendritic cells; DC in lamina propria can also directly capture antigen with their cytoplasmic processes extending between the enterocytes.
In PP, the antigen presentation to Thelper drives B cell to class switching towards the production and secretion of IgA at the lamina propria and in intestinal lumen as defensive effector mechanism (Figure 3). Alternatevely, antigen-loaded intestinal DCs migrate to the mesenteric lymphnode inducing a subsequent antigen-specific T cell and B cells activation; then, the migration of activated B lymphocytes ensures the local production of IgA and also a response even in mucosal surface at a distance (Figure 4).



Similarly, the ability of nasal immunisation to confer an immune response both locally and at distal sites has been demonstrated, by trafficking of cells away from the bronchus-associated lymphoid tissue.
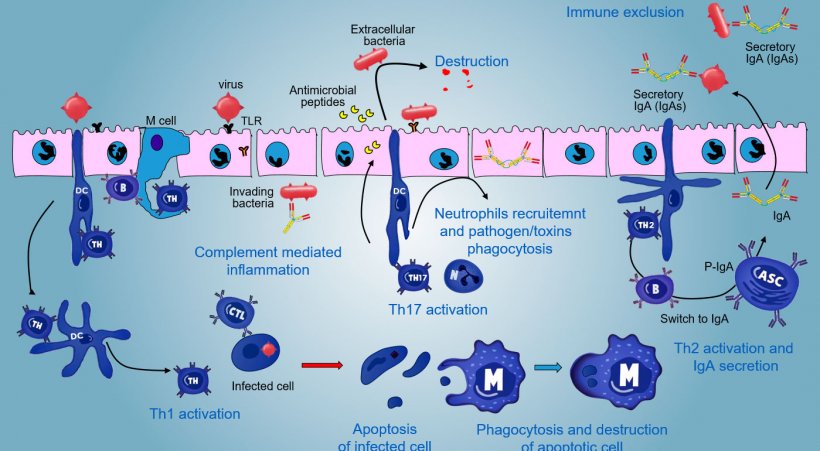
In effector sites, the immune response varies depending on the pathogen to be faced (Figure 5).
In case of intracellular pathogen infection, a TLR and NODs mediated inflammatory response occurs with a subsequent activation of Th1 mediated immune response and activation of NK cells and cytotoxic T lymphocytes able to kill and destroy infected cells; this response could be associated with an efficacious B activation and surface antibodies secretion.
γδT IE lymphocytes are involved in an early eliminaton of infected cells or in the secretion of AMP as well as in the intestinal omeostasis.
A Th17-mediated response plays a critical role in protecting mucosal sites mainly against extracellular bacteria, bacteria and fungi, but also against viruses, in the lamina propria of intestine where these lymphocytes are abundantly present. During an inflammatory response, Th17 lymphocytes activated by IL-23, secrete IL-17 and IL-22, cytokines driving the production of antibacterial peptides and the recruitment and activation of inflammatory neutrophil granulocytes (Figure 5).
In lung, protection against pathogens involves the production of inflammatory cytokines (TNFa, IL-1β and MIP-1α and MIP- 1β), and recruitment of neutrophils, macrophages and lymphocytes such as CD4 T cells , CD8 T cells and γδT lymphocytes.
The production and secretion of IgA secretory antibodies, is a main effector mechanism of mucosal immunity (Figure 6).
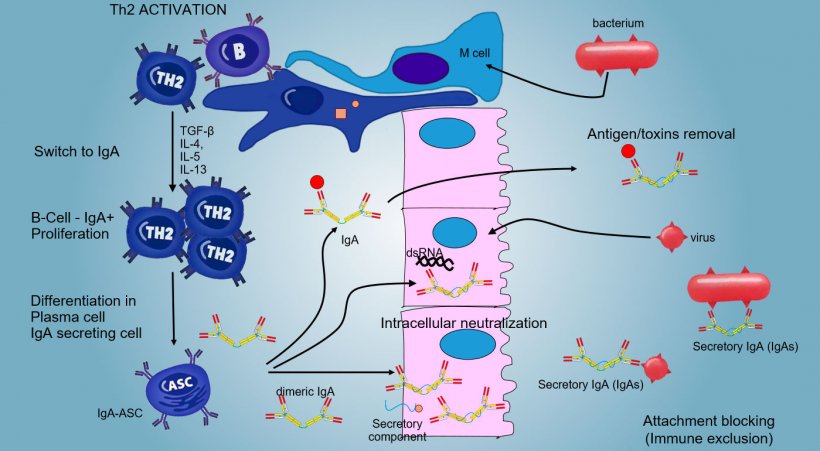
IgA predominate in all surface and mucosal secretions; a consistent secretion of IgA occurs by plasma cells present in lymphoid tissue or in mucosal lamina.
In intestinal PPs, activation of B lymphocytes generates IgA secreting Plasma cells. IgA are mainly secreted as IgA dimers in which two monomer are joined by a J chain and linked to secretory component that protects s-IgA from the action of the intestine proteolytic enzymes. IgAs avoid adherence of viruses and bacteria to epithelial cells (immuno-exclusion mechanism), are able to bind to viral proteins within the cell and block replication (intracellular neutralization) as well as to bind antigens penetrated into the mucosa and to transport them through the epithelium to eliminate them in the lumen (antigen removal).
The "switching" towards IgA is mainly regulate by Th2 lymphocyte activation with production of TGF-β, IL-4, IL-5, IL-13 cytokines. The secreted IgA dimer binds to a receptor (pIgR) on the basal surface of the epithelial cells. The complex is internalized into the cell and, before to migrate across the luminal surface, the receptor is cleaved and the IgA remain to bound to the receptor residue (secretory component).
At the respiratory level, IgA predominates in the upper districts (nasal cavities, pharynx, larynx, trachea, bronchus) while in the inferior districts (bronchial and alveolar) there is mainly a systemic immune response with the involvement of tributary lymph nodes and predominant secretion of IgG.






