Introduction
Despite its scarce use in commercial artificial insemination programs (used only in 1% of all inseminated sows) nowadays no one questions the sanitary, economic, and productive advantages of cryogenically preserved semen with respect to fresh or refrigerated semen. Advantages are inherent to the fact that the semen can be conserved for years submerged in liquid nitrogen (-196°C) without endangering the spermatic quality (Figure 1). This allows for the evaluation of the presence of pathogens in order to eliminate contaminated doses, avoiding the transmission of diseases. It makes the creation of “sperm banks” possible in order to fulfil the needs of farms when the mobility of the animals is not an option, a common situation among the swine species and usually related to infectious outbreaks. Lastly, it foments the international commerce of swine semen. These important advantages encourage scientific research to improve the productivity of cryogenically preserved semen.
 |
 |
| Figure 1. Liquid nitrogen tank used to conserve weekly doses at -196ºC. Detail of the doses stored inside. |
Present situation regarding cryogenically preserved sperm
Traditionally, cryogenically preserved sperm has been considered to be less efficient than fresh or refrigerated sperm. The need to use a large number of spermatozoa per dose for insemination (between 5.000 and 6.000 x106) together with an intrinsically lower fertility rate (20% less births and 2 piglets less per litter) relegated it almost exclusively to very specific genetic modification programs (Johnson y cols., 2000). The recent advances in thermal physical knowledge that characterize and differentiate swine spermatozoa, that have allowed for the design of new cryogenic preservation protocols, along with the development of new insemination procedures, have been key to improving the performance of cryogenically preserved sperm (Roca and cols., 2006b). among the improvements introduced in cryogenics, those that stand out are (1) the substitution of the classic and not very cryogenic 5mL straws used for storing and freezing the spermatozoa, for the 0.5mL straws, or FlatPakcs (Figure 2) that allow for a more homogenous freezing. (2) The adaptation of the freezing and defrosting speeds to the permeability of the sperm membranes; and (3) The use of horizontal bio-freezers (Figure 3), that allow for the uniform freezing of a large number of sperm doses (Roca and cols., 2006a). as far as new insemination procedures, the so called deep intrauterine insemination has demonstrated its efficiency upon obtaining 75-80% birth rates with more than 10 piglets born per litter, inseminating with a dose of just 1.000-2.000 x106 frozen-defrosted spermatozoa (Roca and cols., 2003; Bolarín and cols., 2006); which, additionally, increases the profitability and yield of the semen donor boars upon obtaining a larger number of insemination doses per ejaculation (between 30 and 40 doses).
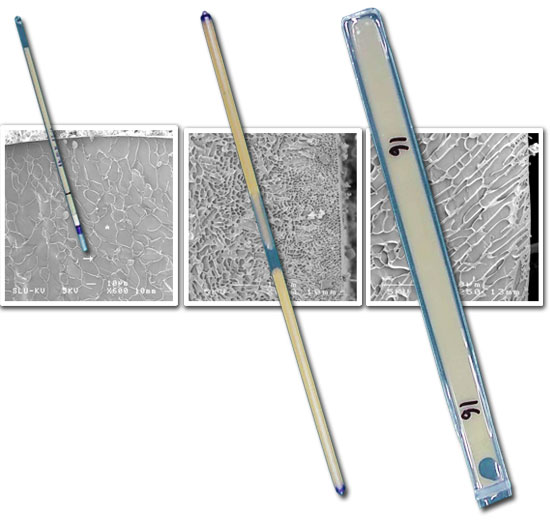
Figure 2. Images from a cryo-microscope showing the cryogenic differences between the 0.5 mLm, 5mL, and flatpack straws used to store and freeze swine sperm (Erikson and cols., 2001 Thierogenology; Ekwall and cols., 2007 Thierogenology; Hernandez and cols., 2007 Cryobiology)

Figure 3. Horizontal bio-freezer that allows for the uniform freezing of a large number of straws (1) Detail of the racks with straws (2) and the availability of a rack in the interior of the bio-freezer chamber (3).
Future possibilities for cryogenically preserved sperm
The advanced mentioned before allow us to use the cryogenic preservation of sperm efficiently in artificial insemination programs. However, in order to universalize its use some progress is still needed, among that being to simplify the freezing process and guarantee that the insemination is performed moments before ovulation. The actual freezing process is laborious and time consuming (+4-5 hours), which limits the number of labs that practice it and it increases the price of the sperm dose (with a cost at least 2-3 times higher than those of fresh or refrigerated semen). As far as the insemination-ovulation interval is concerned, the longer the interval, the less fertile (Figure 4). Improving the initial detection of oestrus and controlling, with a certain precision, its duration, is key to an efficient use of cryogenically preserved swine semen. It is known that ovulation in sows is produced two-thirds of the way into oestrus (Soede and Kemp, 1997). Said knowledge allows for the possibility of inseminating sows within 6-8 hours before ovulation, which facilitates obtaining a labour with birth rates very similar to those obtained with fresh or frozen semen, using cryogenically preserved semen. (Bolarín and cols., 2006).
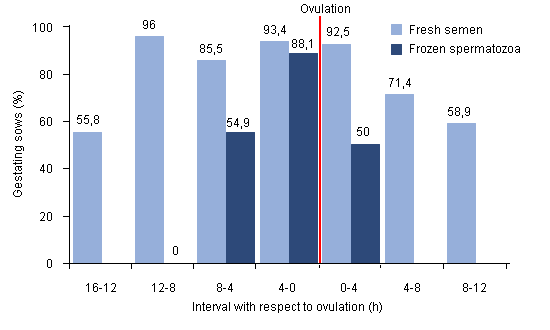
Figure 4. Fertility of fresh semen and cryogenically preserved semen in sows inseminated at different times, with respect to ovulation (Wabersky and cols., 1994 Theriogenology).




