Disease surveillance in replacement gilts
Replacement gilts literally are “new blood” for herds, being essential for sow farm productivity, as they provide genetic advancement and maintain parity distribution. Nevertheless, replacement gilts are also perceived as a risk for pathogen entry (even to the most biosecure farm) due to their frequent introduction into recipient sow herds, which occurs year-round. Several pathogens of great importance, including Mycoplasma hyopneumoniae (M. hyopneumoniae - usually referred to as Mycoplasma), are mainly transmitted through direct contact between infected and susceptible pigs. Therefore, biosecurity practices that include varying isolation lengths and surveillance testing are routinely implemented in replacement gilt populations to aid disease prevention. In general, surveillance protocols are designed to accurately assess the health status of a population and to detect specific pathogens of interest in an early and active manner. Thus, disease surveillance is the backbone of a solid biosecurity program.

Current strategies for M. hyopneumoniae surveillance and their potential limitations
For Mycoplasma surveillance, individual blood samples and pen-based oral fluids are commonly collected and tested for detection of antibodies and genetic material, respectively. Blood samples and oral fluids have been the most utilized sample types to date, largely due to ease of collection, and the fact that the same sample types can be used to target multiple pathogens, such as porcine reproductive and respiratory syndrome virus and porcine epidemic diarrhea virus, for which gilts would also need to be tested for. Using blood samples and oral fluids for Mycoplasma testing maximizes the use of time and effort in the surveillance program. However, what may look as advantages for the use of blood samples and oral fluids for Mycoplasma surveillance, has led to becoming accustomed to employing clinical specimens that can be suboptimal for detection of M. hyopneumoniae, especially during the first weeks post-infection.
On the other hand, several studies in recent years have pointed out that Mycoplasma detection in live pigs is extremely sensitive to time after initial infection and the variation in clinical specimens. Such information poses several questions regarding the accuracy of current surveillance protocols and their ability to detect a recent Mycoplasma infection in a naïve gilt population.
In an effort to evaluate the challenges of Mycoplasma detection under similar conditions to those encountered in the field, our research team designed a study directed at recreating common surveillance protocols for gilt testing prior to sow herd introduction. Other samples types and assays that can be considered state-of-the-art for M. hyopneumoniae detection were also included in the study.
A model of natural transmission of Mycoplasma among gilts
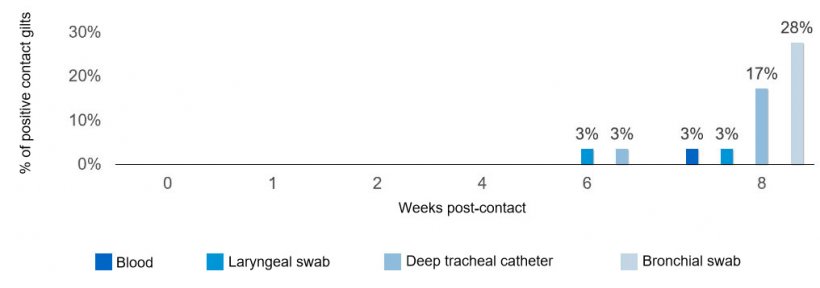
A naturally M. hyopneumoniae infected gilt was introduced into a pen and was housed with group of naïve age-matched gilts (n=30 total) for eight weeks. The naturally infected gilt was in the early stage of infection and her identification was changed after introduction (that way not even researchers were able to discern between naïve and infected gilts and sampling was not biased). Samples were collected from all gilts various weeks post-contact (Figure 1). At each sampling event, deep tracheal catheters, laryngeal swabs, and blood samples were collected from each individual gilt, along with a pen-based oral fluid sample. In addition, individual bronchial swabs were collected at the end of the study to confirm infection. Presence of M. hyopneumoniae specific antibodies was assessed in blood samples using ELISA, whereas the detection of genetic material was evaluated in all other samples by real-time PCR.

No surprises in Mycoplasma detection
As expected, transmission of M. hyopneumoniae among naïve gilts occurred slowly and most clinical specimens in live naïve pigs resulted negative for the pathogen, especially during the first weeks post-contact with the infected gilt (see Figure 2). Only after eight weeks, 8 of the 29 of the naïve gilts became infected with Mycoplasma. At six weeks post-contact, one naïve gilt became positive for Mycoplasma in deep tracheal catheters and laryngeal swabs and it was not until eight weeks post-contact when this newly infected gilt became antibody positive. It is important to note that oral fluids remained negative for Mycoplasma detection for the entire eight weeks of the study, regardless of the fact that one positive gilt was part of the sampled group.
Although M. hyopneumoniae was detected throughout the entire study using deep tracheal catheters and/or laryngeal swabs in the naturally infected gilt, seroconversion was delayed and only first identified six weeks post-infection.
Opportunities to optimize Mycoplasma surveillance protocols
One message is very clear from the results of this investigation: The timing after exposure and the type of sample employed for M. hyopneumoniae testing are crucial and will significantly influence the capability to detect this pathogen. Samples collected from the lower respiratory tract, for example, deep-tracheal catheters, offered the highest sensitivity among sample types. Knowing that a comprehensive surveillance program is key to prevent sow herds disease outbreaks, it is critical for veterinary professionals to revisit existing protocols and to redesign them based on the most current diagnostic information. Traditional surveillance protocols should not become a tradition. Swine practitioners can rely on field-based research for improved approaches to Mycoplasma disease prevention.
This study was funded by the Minnesota Pork Board and the Pork Checkoff.








