Introduction
Porcine reproductive and respiratory syndrome virus (PRRSV) is one of the most economically significant pathogen in swine industries of many countries. It is reported that annual economical losses due to PRRSV are $641 million in USA (Holtcamp, et al. 2011) and ¥28 billion in Japan (Yamane, et al. 2010). It has been shown that PRRSV can be controlled and possibly eliminated in individual farm basis via multiple strategies, such as gilt acclimatization (Dee, et al. 1994), depopulation/repopulation (Dee, et al. 1994), mass vaccination (Dee, et al.1996), test & removal (Dee, et al. 1998), herd closure with/without intentional exposure (Toremorrell, et al. 2000) and so on. However, re-infection of new strains of PRRSV is still frequent, and area spread of the virus is the biggest challenge we have been facing on in regards to PRRSV control today. This article summarizes recent research information on transmission and biosecurity of PRRSV. It also indicates the significant importance of area regional approach on control/elimination of PRRSV in future.

Transmission and biosecurity on PRRSV
For the past 10-15 years, a number of research has been conducted in the area of PRRSV transmission and biosecurity. The results from those studies provided us with a reasonable knowledge to understand how PRRSV can be transmitted and how we can prevent it. Figure 1 shows a summary of known transmission routes of PRRSV with selected published references, along with field-practiced biosecurity interventions in order to reduce it’s risk.
Table 1. Known routes of PRRSV transmission
| Transmission routes | References | Biosecurity interventions |
| Pigs and semen | Yoon et al. 1993, Christopher-Hennings et al. 1995 | Quarantine and testing |
| People | Otake et al. 2002, Dee et al. 2012 | Shower-in/out |
| One-night down time | ||
| Coveralls and boots | Otake et al. 2002 | Changing coveralls and boots |
| Needles | Otake et al. 2002 | Changing needles |
| Fomites (lunch boxes, shipping containers, etc) | Dee et al. 2003 | UV path-box, fumigation room, double-bagging, etc |
| Insects (mosquitoes and house flies) | Otake et al. 2003 | Insect screen |
| Transport | Dee et al. 2004 | Wash, disinfect and dry |
| Manure | Linhares et al. 2012 | |
| Aerosol | Pitkin et al. 2010, Otake et al. 2012 | Air filtration |
Airborne transmission of PRRSV and air filtration
Recently, long distance airborne transport of PRRSV was reported under field condition (Otake, et al. 2010). In this study, a mixed infection of 3 PRRSV variants (1-8-4, 1-18-2, and 1-26-2) was established in a source population of 300 growing pigs. Over 21-day period, air samples were collected from the source population and at designated distances from the herd. Samples were tested for PRRSV by RT-PCR. In exhaust air from the source population, PRRSV was detected in 21 of 21 air samples collected. Five of 114 (4.4%) long-distance air samples were positive for PRRSV, and they were collected at 2.3, 4.6, 6.6 and 9.1 km from the herd (Figure 2). All contained infectious virus and were more than 99.2 % homologous to PRRSV 1-8-4. These results indicated that long distance airborne transport of infectious PRRSV can occur under field conditions.
Fig. 2. Aerial map depicting the location of the sampling points where the 5 PRRSV-positive air samples were recovered in relationship to the source population. All 5 samples contained infectious virus as determined by swine bioassay. Titers of infectious PRRSV (expressed in units of TCID50/mL) present in each of the 5 samples are noted at each of the 5 collection points.
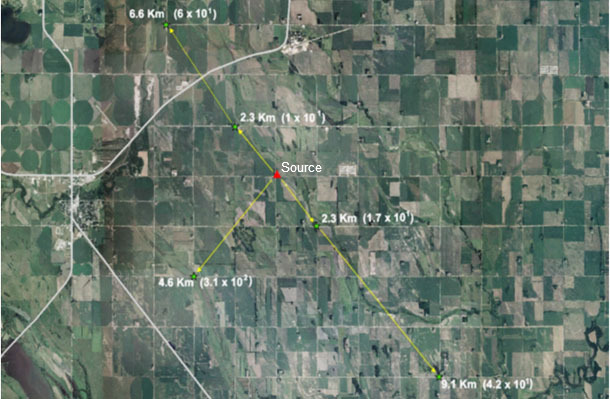
Otake, et al. 2010
In order to reduce the risk of airborne transmission of PRRSV, an application of air filtration was attempted. Dee and others demonstrated the efficacy of air filtration under controlled field conditions (Dee et al. 2010). In this study, all types of filters successfully prevented airborne transmission of PRRSV in the production region model (Figure 3). The study also revealed meteorological risk factors that were associated with probability of airborne transport of PRRSV, which included cool temperatures, the presence of the pathogen in the source population, wind direction, low sunlight levels, winds of low velocity in conjunction with gust and rising humidity and pressure. These data provided better understanding of aerobiology of PRRSV and validated several filtration technologies for protecting susceptible population against the airborne challenges of PRRSV.
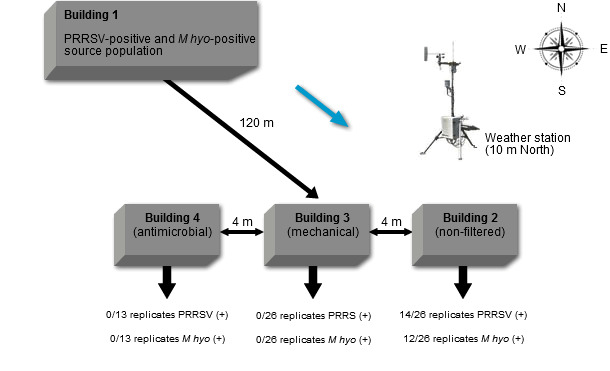
Fig. 3. Placement of buildings within the production region model during years 1 and 2 of the study. Buildings 1, 2 and 3 were used throughout the 2-year study period while building 4 was only used during year 2. Building 1 served as the source of PRRSV and Mhyo-positive bioaerosols. Buildings 2 (non-filtered control), 3 (mechanical filtration) and 4 (antimicrobial filtration) were placed 120 m down wind to enhance their exposure to bioaerosols transported via prevailing winds. Note placement of Weather station 10 m north of building 2. Dee, et al. 2010

The efficacy of air filtration was also proven in large commercial sow farms in US (Dee. et al. 2012). This study showed that the likelihood of a new PRRSV infection was significantly higher (p < 0.01) in non-filtered herds versus filtered herds. The odds of a new PRRSV infection were 8 times higher (p < 0.01) before filtration than after filtration. The median time to new PRRSV infections in filtered herds (30 months) was significantly lower (p <0.01) than in non-filtered herds (11 months). In conclusion, filtration significantly reduces the risk of new PRRSV infections in large commercial sow farms (Figure 4).
Figure 4: The likelihood of a new PRRSV infection was significantly higher (p < 0.01) in non-filtered herds versus filtered herds. The odds of a new PRRSV infection were 8x higher (p < 0.01) before filtration than after filtration. The median time to new PRRSV infections in filtered herds (30 months) was significantly higher ( p < 0.01) than in non-filtered herds (11 months).
| Category | # farms | Cumulative days | # New infections | Interval | Infections per farm |
| Filtered | 24 | 16,593 | 8 | 2074 d | 0.33 |
| Non-filtered | 33 | 29,533 | 89 | 336 d | 2.7 |
Dee, et al. 2012
Area regional approach on control/elimination of PRRSV
Due to the fact that area spread of the virus is the biggest challenge of PRRS control we have been facing on today, area regional approach on control/elimination has been recently initiated. More than 25 regions in North America initiated such activities and have been making some degree of progress depending on each region (Morrison, 2012). Not only in North America, but such initiatives also have been made in other countries including Netherlands (Duinhoff, 2012), Korea (Jeong, et.al 2013), and Japan (Otake, et al 2013). So far, all those activities/programs are totally swine producer-driven at voluntary basis, along with collaboration of universities or industrial partners. Area regional approach requires a great deal of communication and information sharing among the participant producers, vets, and all other supporters within that region. It indicates that area regional approach is one of the most important key for PRRSV control/elimination in future.
Conclusions:
PRRS is economically significant disease. Area spread of PRRSV is still frequent event; therefore, biosecurity is one of the most important key component of PRRS prevention. Transmission routes of PRRSV have been scientifically proven already, and biosecurity interventions should be practiced based on those science. A risk of airborne transmission of PRRSV is real, and air filtration can significantly reduce its risk. Area regional approach on PRRSV control/elimination indicates our direction where we should move forward in future.