Single animal treatment
Injection treatment can be done with many different medications depending on agents: E. coli, Lawsonia intracellularis and Brachyspira pilosicoli, each have their own treatment which is most effective and this should therefore be adjusted to each herd.

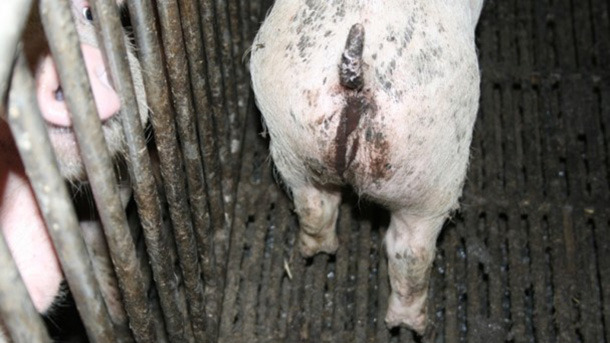
It can be difficult to find the relevant animals which need treatment. The easiest way is to aim for those which are dirty on their hind parts (picture 1) or maybe have a red rectal area. However research shows that it will only be possible to find approximately one fifth of all animals requiring treatment this way. In other words, there are a lot of animals which are not found and this has an impact on productivity, health and animal welfare.
Another solution is of course to wait until the faeces from the animal can be seen, but in a pig production system with many pigs housed this is not practically possible. However, what should be done is that the animals which are found to have watery to mushy faeces should all be treated. This also applies even though the pig appears to have a nice body condition because such animals can after all have a very high secretion of disease level with various intestinal infections. On the other hand, unthrifty pigs without diarrhoea can also have intestinal infections and should be treated accordingly. As a general rule, treatment should always last minimum three days but until the animal is well.
Another way of doing single animal treatments is to use hospital pens. The animals which require treatment are gathered in such pens and here they are injected, alternatively treated via feed or water medication. Experience from clinical practice shows that peroral medication has a better effect than injection medication. At the same time, this means that when pigs are gathered in such pens, they get more air and space, and at the same time it is possible to give them better feed such as slightly coarser ground feed containing more barley and less protein.
Batch medication
Based on previous explanations, it also appears that the most correct way of treating is actually batch medication, and this is especially because there is a very high number of the pigs which actually have a subclinical intestinal infection. This is well-documented for Lawsonia but also applies to the other intestinal diseases.
Administration at batch level can be done via feed or water. In Denmark, as in many other countries, it is most common to do it via water medication and to wait until the pigs have clinical signs. When beginning diarrhoea droppings are seen, medication is started. However, a number of research projects have shown that it is very difficult to find the correct time to treat. The farmer will assess that some of the units should not be treated, and 30-40% of the pigs will turn out to have diarrhoea and a just as high number will have intestinal infections. On the other hand, there could be units where the farmer assesses that there is diarrhoea and 30-50% of the pigs do indeed have diarrhoea but when samples are taken from such units, in some cases infections will not be detected in the intestines. Therefore in such cases the antibiotic treatment should not be necessary.
This means that it has been necessary to find some new methods to find the correct time of treatment and therefore sock samples are now used in Denmark (picture 2). The idea is that through repeated sock samples, the correct time of treatment of the animals can be found. Preliminary results from a research project at University of Copenhagen indicates that this might be a solution for the future. However, faster PCR tests needs to be developed and preferable they should be used in the stables, so called pen-side tests. For now the sock-samples need to be submitted to a laboratory and is used in practice for determine cause of diarrhoea and timing of treatment.

At present it is not possible to do this on a day-to-day basis, but this means that medication must either be done based on outbreak of clinical diarrhoea or alternatively at a more fixed time. For example, this can be determined by using serological or quantitative PCR profiles which show when the pigs seroconvert or are infected by Lawsonia. Similar quantitative PCR can be used for E. coli and Brachyspira pilosicoli. Alternatively based on our knowledge of inhomogeneous pigs occurring at a certain point during the growth period and then treatment should be placed immediately before this. The challenge here is that there is a variation between batches which can be difficult to take into account.
In any case, the treatment and choice of medication should be done based on resistance testing. This is of course not possible with regard to Lawsonia, but it is possible for both E. coli and the Brachyspira types and it has especially become relevant for the latter as many European countries now have problems with resistance both towards swine dysentery and Brachyspira pilosicoli.



