Introduction
This case study took place in the summer of 2010 in the Midwest of the United States of America. The farm involves a small farrow-to-finish operation where all pigs are finished on the same site. The farm consists of a gestation and farrowing barn with a capacity of 150 females, a “double L” nursery facility with a capacity of about 400 pigs and a finishing barn with a capacity for 800 pigs. Additional miscellaneous barns were used intermittently including a small, solid floored flush gutter style building that housed 13 boars. At the time of this case, the farm inventory consisted of approximately 75 sows, 700 growing pigs, and 13 boars. The boars on this farm are utilized as if they are a stand-alone boar stud, as semen is both utilized for their own females and sold domestically and internationally.

The regular herd veterinarian was contacted by the farm with the request to sell semen from 1 boar to a customer in the Far East. Knowing a boar who produces semen for exportation (donor boar) must meet the unique health requirements for each consignee market, the veterinarian contacted the United States Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) area veterinarian-in-charge for guidance with specific testing requirements for exportation. In this case, the semen was destined for Japan which requires the donor boar to be tested with negative results within 60 days prior to collection for export for: Vesicular Stomatitis, Aujesky’s disease, PRRS, Brucellosis, Leptospirosis, and Tuberculosis.
Tuberculosis testing antemortem relies on a delayed type hypersensitivity reaction due to an intradermal injection of a licensed USDA purified protein derivative (PPD) tuberculin product which must be obtained through a State animal health official or an APHIS-VS Area Office. Furthermore, this testing must be performed by a licensed and accredited veterinarian.
Prior to the tuberculosis test, the farm was instructed to withhold from treating the boar with any medications or anthelminthic and from administering any vaccinations, if possible, without jeopardizing the health and welfare of the animal. Any of these agents may temporarily affect the boar’s immune system and thus influence the result of the tuberculin test which is based on an immunological response to the injected tuberculin. Also, required prior to the farm visit are an official Tuberculosis Test Record VS Form 6-22 and a disposable 1.0cc plastic tuberculin syringe and a 26 gauge 3/8-inch-long needle for each injection.
The tuberculosis testing protocol involved injecting 0.1ml of Mycobacterium bovis PPD tuberculin into the dorsal surface of the base of the right ear and 0.1ml of Mycobacterium avium complex (MAC) in the same location on the left ear. The needle was directed between the superficial layers of the skin and inserted to its full length. Injections resulted in small blebs in the skin at the end of the needle. At the time of injection, any scars, defects, insect bites, or other skin abnormalities were noted which could later be confused with an injection response. The test is read 48 hours after injection, as compared to 72 hours in other mammalian species, for any evidence of induration or inflammation associated with the injection site. The 3 possible outcomes when reading the injection sites classify the animal as a positive, suspect, or negative reactor. Any swelling greater than 3mm in diameter is considered a positive reactor, while changes resulting in less than a 3mm nodule are considered suspect reactors and no reaction is categorized as a negative reactor.
Farm visit and testing
Prior to tuberculosis testing, a routine herd health visit was performed. In general, herd health and production parameters were good for this particular farm and had been for several months. No signs of clinical disease were evident in any stages of production, including the boars.
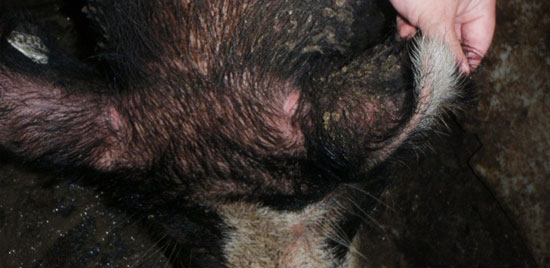
fig. 1
At this time the boar was restrained with a snare, positively identified, and injected with the tuberculin antigen as previously described. The injections were read 48 hours later with the M. bovis showing no changes while the M. avium had a palpable increase in skin thickness (as shown in Figure 1) and was recorded as a suspect reactor to the M. avium tuberculin. At this time the state veterinarian was consulted and the farm was provided with the following options: the suspected reactor could be marketed to a Food Safety and Inspection Service (FSIS) regulated slaughter facility, the reactor could be euthanized and have a complete necropsy performed looking for related lesions, or the farm could quarantine the entire boar facility, test all the other boars on the farm, and retest this boar 60 days later. The farm opted for the quarantine, test, and retest option due to the high economic value of the suspect reactor.
The following week, all other boars underwent tuberculosis testing following the same protocol, with the exception of one boar which was marketed at an FSIS slaughter facility. Upon evaluation of the injections, 10 boars had no reactions while 1 boar had a raised and hyperemic reaction to the M. avium injection site and was determined to be a positive reactor. At this time, the state veterinarian was again consulted and the same options were provided to the farm with the exception of both reactors to be marketed, euthanized, or quarantined and retested. The farm again chose to place the facility under quarantine (could not move any of the boars to another location or market any semen) and to retest both boars 60 days from the last test.
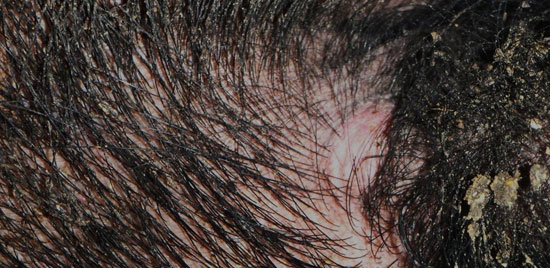
fig. 2
The 2 reactors were retested 60 days later using the same protocol with the exception of switching the ear base side for each isolate. Each boar was classified as strong reactors to the M. avium injection (figures 2 – 4). After 2 positive tests, the second tested boar was submitted for a full necropsy at a state veterinary diagnostic laboratory. A thorough and complete post-mortem evaluation revealed no gross or histological lesions consistent with clinical disease. The initial reactor was relocated to a quarantined site and continued to provide semen for domestic services.
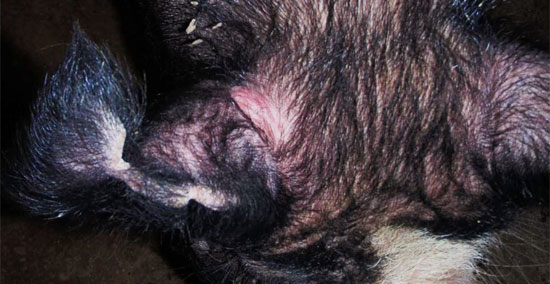
fig. 3
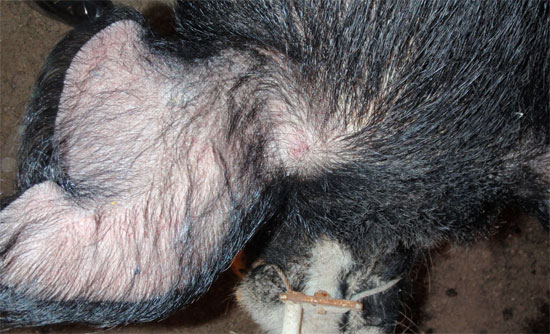
fig. 4

A retrospective epidemiological investigation revealed the 2 reactors were the only animals on the farm whom were housed at an offsite quarantine facility. The initial reactor was in the facility 1 year prior to testing and the second had been there several months prior. The facility was visited and the barn was found to be open to the environment, housed many birds, and the pens were unsatisfactorily cleaned between down periods.
Despite the lack of lesions, the top differential for the 2 reactors are true infections with M. avium. False positive results are less likely given the 2 reactors and the circumstantial evidence linking the 2 reactors for possible sources of infection and modes of transmission. It is not uncommon for pigs to have been infected and subsequently clear the infection without permanent lesions or to harbor the organisms without evident lesions (Martín-Hernando MP, 2007). The quarantine facility was implicated as a potential source of infection which may have been the direct source for both boars or the source for the initial boar with horizontal transmission to the second boar as they had nose-to-nose contact on the farm. Recommendations to the farm were to improve biosecurity practices across all production phases and to find a more suitable quarantine facility which could properly exclude wildlife from the animal housing area.
Discussion
Tuberculosis is not routinely tested for in the United States due to the low prevalence of disease in today’s herds. According to the FSIS Animal Disposition Reporting System, in 2008, of 115 million carcasses slaughtered just 0.02% had tuberculous lesions. However, there have been recent outbreaks of tuberculosis in commercial systems which have resulted in significant economic losses. Epidemiological investigations of these cases implicated contaminated saw dust bedding, cooling systems, and contaminated feed (Daniels CS et al, 2009; Álvarez J et al, 2011; Lower AJ, 2011). Pigs are known to be susceptible to infection to tuberculosis species by a variety of methods including: feeding of unpasteurized dairy products, direct contact with cattle, feeding offal and uncooked garbage, access to dirt in which poultry had previous contact, contact with wild birds, and direct transmission between infected pigs (Thoen CO, 2006). Contaminated products and premises are a significant long term threat to swine due to the inherent resistance of mycobacterial organisms to environmental degradation.
Of the 3 tuberculosis species (Mycobacterium tuberculosis, Mycobacterium bovis, and Mycobacterium avium complex) swine are susceptible to, MAC is the least likely to be a zoonotic threat (Thoen CO, 2006). It typically only causes disease in immunocrompromised patients (Falkinham JO, 2003). The MAC is composed of 8 individual bacterial species and numerous subspecies with variable pathogenic characteristics, environmental distribution, and host preference (Neuman EJ, 2010). In the pig, there typically are no evident clinical signs with the only presentation being carcass condemnation at slaughter. Affected older animals tend to waste away despite adequate feed intake. Definitive diagnosis can be made only after isolation, identification, and typing of the bacteria. Prevention of disease is by avoiding exposure of swine to infectious mycobacteria. All swine, bedding, and feed products should be protected from birds and other tuberculous harboring mammals.
Furthermore, this case serves as an example of the possible consequences of testing for reportable organisms which may not necessarily be relevant clinically or pose a threat to production or zoonosis. This farm requested such testing to gain access to international markets as an added source of revenue. However, it resulted in losing 2 productive and valuable boars in addition to the cost of testing.







