Monitoring the presence of PRRS virus on sow farms is used to classify the status of the farm. The classic and most widely used sampling and classification system is based on that developed by Holtkamp, et al (2011) with blood samples taken once a month for several consecutive months. According to this protocol, a farm is considered:
- Negative when the breeding sows are ELISA negative
- Positive stable when no viraemic pigs have been detected by PCR at weaning for at least 90 days
- Positive unstable when the virus has been detected in any tissue without achieving a minimum of 90 days of negative PCR results to be considered as positive stable.
At the same time, monitoring can be used to assess the effectiveness of control measures put into place following a reproductive PRRS outbreak . In this case, the initial objective is to achieve stability at farrowing, meaning the absence of virus in all tissues from newborn piglets. The second objective is to achieve stability at weaning; and finally, if there is a nursery stage on that farm, to achieve the absence of the virus in the samples taken at the end of the nursery.

New types of samples for PRRS monitoring
After a few years of exclusively using blood as the only sample type in PRRS monitoring, new sampling systems for larger populations were developed such as oral fluids collected by ropes which is widely used in nursery and fattening pigs. More recently, new techniques for PRRS virus detection from aggregate samples of testicular fluid and tails collected during processing have been studied with good results, Vilalta et al (2018). The results published are better with processing fluids from testicles than from tails. The use of processing fluids makes it possible to sample more animals, and more frequently, which is very useful for detecting PRRS virus when the percentage of carrier animals at birth is low. When the percentage of piglet castration is low, the practical application of this technique is not possible. In addition, the restrictions on routinely performed tail docking as per European animal welfare regulations also limits the use of processing tail fluid as the sample of choice.
Based on these guidelines, objectives, and conditions, the team from the Grup de Sanejament Porcí in Lleida, Spain, developed a project to evaluate a new monitoring technique based on the collection of aggregate samples from parts of dead animals. The samples are stored frozen and sent to the laboratory with a determined frequency. The collection and analysis of this type of aggregate and continuous set of samples allows us to determine the moment from which no virus is detected in the tissues of the dead animals.
Following the first results, it was suggested that collecting pieces of tongue from stillborn piglets is a good system for determining if there is virus present in the piglets at birth.
Tongue tissues from dead pigs as a diagnostic sample
The system is based around collecting at least 2 cm-pieces of tongue, storing them in bags or containers in the freezer, and sending them at the desired frequency (monthly or by farrowing batch). This system has shown good results and many advantages. The tongue was chosen because it is generally a cleaner sample than the tail, it provides an adequate amount of fluid, and in the case of stillborn pigs, other elements such as saliva and amniotic fluid are also present (Photo 1). In addition, it does not require specific technical training so farm workers can take the samples themselves.

Photo 1. Tongues from dead nursing piglets.
For lactation, nursery or fattening, the same sampling system is used; a portion of the tongues is obtained from the animals that died during these phases in the period to be studied. In these cases, the samples are stored separately in three containers, according to the phase in which the death occurred: beginning, middle or end of nursery or fattening.
This aggregate sample is stored frozen in bags or containers until sent to the diagnostic laboratory. There it is processed to extract the fluid from the traces of liquid present in the tongues.
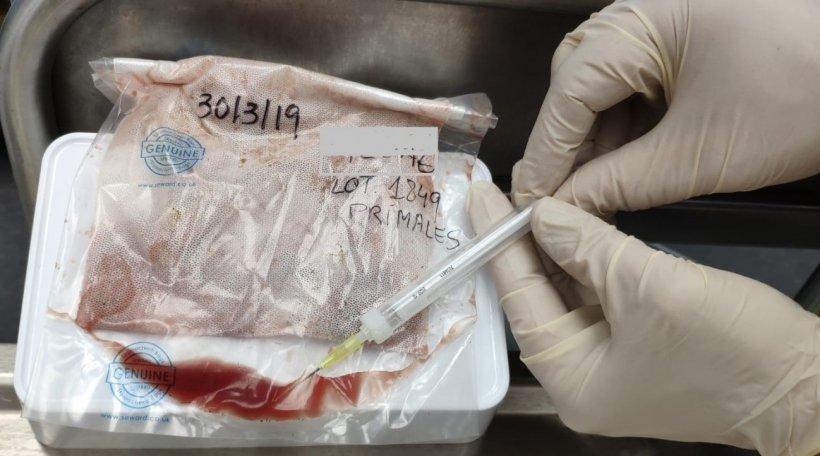
Photo 2. Fluid collection from the tongue of stillborn piglets after thawing.
With this fluid, a PCR is performed to determine the presence or absence of the virus. It has also been possible to sequence several portions of the viral genome using the Sanger sequencing technique.
The basic requirements for this sampling system are: a freezer available on the farm and a sufficient number of animals to sample during the time period to be studied.
The main advantages of this sampling system are

- It usually allows more animals to be sampled with more frequency. Routine collection of the tongues of all stillbirths and dead animals during lactation can be implemented.
- Sampling does not require complicated technical training. Farm employees are able to collect these types of samples themselves.
- It eliminates the stress associated with taking samples from live animals.
- The sensitivity and specificity of the technique improves in some cases by targeted sampling and an increased number of animals sampled.
- The quality of Sanger sequencing is usually better than that of oral fluids.
- Finally, the cost of disease monitoring is lower compared to traditional monthly bleedings.
Monitoring PRRS using samples from dead pigs allows us to obtain important epidemiological information in production phases where less effort has been focused on monitoring the virus presence such as at birth in nursery and fattening.