 Merck Animal Health (known as MSD Animal Health outside the USA and Canada) today announced that it has received marketing authorization in the European Union for PORCILIS® M Hyo ID ONCE, the first single-shot vaccine against Mycoplasma hyopneumoniae (M Hyo) infection in pigs for intradermal administration. PORCILIS M Hyo ID ONCE is approved in the 25 member states of the European Union plus Iceland and Norway for the active immunization of finishing pigs to reduce pulmonary lesions and the decrease in daily weight gain during the finishing period due to infection caused by M Hyo.
Merck Animal Health (known as MSD Animal Health outside the USA and Canada) today announced that it has received marketing authorization in the European Union for PORCILIS® M Hyo ID ONCE, the first single-shot vaccine against Mycoplasma hyopneumoniae (M Hyo) infection in pigs for intradermal administration. PORCILIS M Hyo ID ONCE is approved in the 25 member states of the European Union plus Iceland and Norway for the active immunization of finishing pigs to reduce pulmonary lesions and the decrease in daily weight gain during the finishing period due to infection caused by M Hyo.
 The new vaccine differs from the existing PORCILIS M Hyo in that: (1) the new vaccine comes as a single-shot (whereas the existing vaccine is available as a two-shot vaccine), and (2) the new vaccine is approved for use as an intradermal administration (whereas the existing vaccine is approved for use as an intramuscular administration). This new single-shot, intradermal application of the vaccine adds flexibility and convenience in use to the current dosage scheme that has been available in Europe since 2004. Market introduction in the individual countries will depend on the granting of national licenses that have been scheduled for the coming four to six months.
The new vaccine differs from the existing PORCILIS M Hyo in that: (1) the new vaccine comes as a single-shot (whereas the existing vaccine is available as a two-shot vaccine), and (2) the new vaccine is approved for use as an intradermal administration (whereas the existing vaccine is approved for use as an intramuscular administration). This new single-shot, intradermal application of the vaccine adds flexibility and convenience in use to the current dosage scheme that has been available in Europe since 2004. Market introduction in the individual countries will depend on the granting of national licenses that have been scheduled for the coming four to six months.

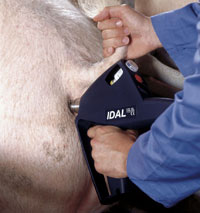
Merck Animal Health already has two other vaccines with a label claim for intradermal use (in addition to an intramuscular claim) - PORCILIS PRRS and PORCILIS Begonia. The company has a needle-free ‘Intra Dermal Application of Liquids’ (IDAL®) device which is used for the intradermal application of these vaccines. Before or in conjunction with the commercial launch of PORCILIS M Hyo ID ONCE the company will introduce an improved IDAL device for intradermal use with all three vaccines.
Infection with M Hyo is known to cause enzootic pneumonia, a chronic pneumonia which can be complicated by opportunistic bacterial infections (such as P. multocida, B. bronchoseptica, S. suis, H. parasuis and A. pyogenes). There is usually an incubation period of two to eight weeks before clinical signs of M Hyo infection are seen. Over the first six to eight weeks after it enters the population there may be severe acute pneumonia, dehydration, heavy breathing, coughing, respiratory distress and fever. This results in increased mortality, as well as a reduced rate of weight gain and lower feed efficiency. As a consequence, M Hyo infection in a pig herd often results in significant economic losses for the farmer.
October 25, 2011 - Merck Animal Health


