Today the U.S. Food and Drug Administration released the 2018 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals, which showed that domestic sales and distribution of medically important antimicrobials for use in food-producing animals increased nine percent between 2017 and 2018. Despite this increase, 2018 is the second-lowest year on record and the overall trend continues to indicate that ongoing efforts to support antimicrobial stewardship are having an impact: sales in 2018 are down 21 percent since 2009, the first year of reporting, and down 38 percent since 2015, the peak year of sales and distribution.
In 2018, 2,374,348 kg of medically important antimicrobials were sold for use in swine, representing 39% of the total sales for all species. For swine, medically important antimicrobial sales were up 17% from 2017 but down 24% compared to 2016.

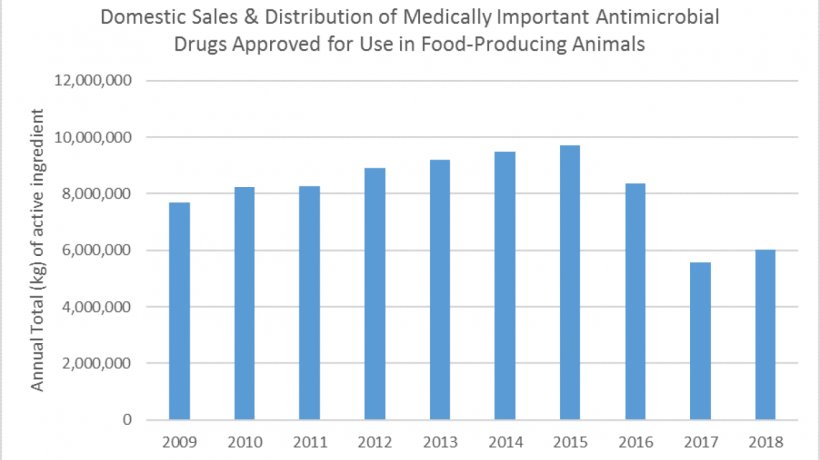
When analyzing the report, readers should consider that sales and distribution information does not represent actual use of the products; however, sales volume observed over time is a valuable indicator of market changes related to antimicrobial drug products intended for food-producing animals. The FDA’s objective is to slow the development of antimicrobial resistance and preserve the effectiveness of antimicrobials for fighting disease in animals and humans while fostering good antimicrobial stewardship practices.
December 10, 2019 - FDA
https://www.fda.gov/





